Overview of Liver Organoid Models
International Journal of Molecular Sciences. 2023 Nov 2; 24 (21): 15921.
Authors: Iqbal W, Wang Y, Sun P, Zhou X.
INTRODUCTION
Various human cell and tissue models have been developed such as immortalized cell lines, xenografts, and organoids derived from differentiated stem cells to overcome the limitations posed by animal models. Every model has advantages and disadvantages; for instance, cells cultured in a conventional 2D system, where cells grow in a monolayer, are easy to maintain and respond well to genetic engineering tools, making them suitable for drug discovery and understanding disease progression. Nevertheless, 2D models lack cell-cell, and cell-niche interactions that are crucial to the functioning of human organs in vivo. These limitations could be addressed by using patient-derived xenografts that essentially recapitulate the heterogeneity and intricacy of the tissue of origin. However, xenografts are expensive to establish and are not suitable for high-throughput screening experiments. This has led to a great deal of interest in organoids, which have been in use for almost two decades largely in the field of regenerative medicine.
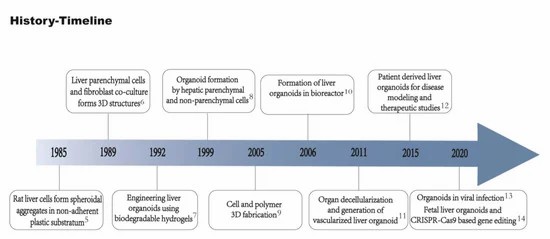 Fig. 1 Timeline of important events in the technology of liver organoids.
Fig. 1 Timeline of important events in the technology of liver organoids.
Adult Stem Cell-Derived Liver Organoids
Ever since the establishment of crypt-villus organoids from LGR5+ stem cells, organoids have been developed from tissue-specific stem cells for various organs using a similar approach. For instance, hepatotoxin (carbon tetrachloride)-induced liver injury causes the Wnt-regulated LGR5+ stem cells to appear in the surroundings of bile ducts. These cells give rise to cyst-like structures when cultured in the presence of Wnt agonist RSPO1. The cells could differentiate into hepatocytes in vitro and repopulate Fah−/− mice upon transplantation. Moreover, EpCAM+ biliary cell-derived human adult liver organoids were found to be genetically stable even after expansion for over a year in vitro; consequently, these models could be used as indispensable tools in regenerative medicine, drug discovery, and understanding liver diseases.
Liver Organoids Derived from Primary Hepatocytes
The ability of hepatocytes to proliferate in vivo has been exploited to generate organoids that expand for months in vitro. In one such study, organoids generated from mouse primary hepatocytes and human fetal hepatocytes formed 'grape-like' structures normally seen in organoids developed from hepatocytes, as opposed to cyst-like structures formed by biliary epithelial organoids. Interestingly, the organoids developed from hepatocytes exhibited regenerative characteristics after partial hepatectomy and had functional similarities to primary hepatocytes. Moreover, hepatic organoids have also been derived from fibroblasts differentiated into hepatocytes by inducing the expression of hepatocyte-specific transcription factors.
hPSC-Derived Liver Buds
The ability of hPSCs to self-renew and differentiate into different types of cells has led to the development of numerous organoid models for liver and multi-organ organoids, where multiple cell types represent different human organs. The methodology that was first used to develop liver organoids in vitro involved the generation of liver buds, where iPSC differentiated into hepatic endoderm cells co-cultured with MSCs and human umbilical vein endothelial cells to emulate liver organogenesis [89]. Interestingly, the cells organized into a three-dimensional liver bud on Matrigel had a gene expression profile similar to mouse embryonic liver buds. The buds were able to vascularize and rescue a liver failure mouse model when engrafted. Later, the same protocol was further improved and liver organoids were developed entirely from iPSC-derived endoderm, mesenchymal, and endothelial progenitor cells.
Cholangiocyte Organoids
Numerous studies have utilized signaling cues to direct the differentiation of hPSCs into cholangiocyte organoids, hepatic organoids, or hepatobiliary organoids having multiple types of cells. The developmental signaling cues have been used to guide the differentiation of hPSCs into definitive endoderm followed by differentiation into definitive endoderm and ultimately into cholangiocytes with the induction of the notch signaling pathway. The cholangiocytes were able to self-organize into organoids with structural similarities to the ductal structures found in the liver and expressed biliary marker genes such as apical sodium-dependent bile acid transporter and cystic fibrosis transmembrane conductance regulator-mediated fluid secretion when embedded in Matrigel.
hPSC-Derived Hepatobiliary Organoids
Multi-cellular hepatobiliary organoids that consist of hepatocytes and cholangiocytes exhibiting bile duct-like morphology have also been developed by several groups, recapitulating the hepatic developmental process of an embryo. These organoids exhibiting the expression of hepatogenic genes and hepatobiliary functions were used to identify the role of JAG1 mutations in the development of liver disease. In another study, iPSC-derived EpCAM+ endodermal cells were used to generate hepatic organoids that were able to expand for over a year and were used to characterize the role of the argininosuccinate synthetase (ASS1) enzyme in citrullinemia type 1 disease. Hepatobiliary organoids have also been generated in a novel 3D suspension culture where the organoids self-organize and form functional bile canaliculi and are thus useful models for studying cholestasis induced by drugs or disease.
Creative Bioarray Relevant Recommendations
| Product/Service Types | Description |
| Human Adult Stem Cells | Creative Bioarray provides a series of human adult stem cells, which are in a non-specific state. |
| Primary Hepatic Cells | At Creative Bioarray, we offer 33 types of human hepatic cells including Human Liver Carcinoma Epithelial Cells, Human Liver Sinusoidal Microvascular Endothelial Cells, Human Liver Tumor-Associated Endothelial Cells, Human Hepatic Sinusoidal Endothelial Cells, etc. |
| Pluripotent Stem Cells | Creative Bioarray offers high-quality and well-characterized ESCs, iPS cell lines, and their derivatives generated from patients and healthy controls. |
RELATED PRODUCTS & SERVICES
Reference
- Iqbal W, et al. (2023). "Modeling Liver Development and Disease in a Dish." Int J Mol Sci. 24 (21): 15921.s