CTC Isolation with Aptamer-Functionalized
Chitosan nanoparticle surface fabricated by electrospray was modified by polyethylene glycol and a DNA aptamer for a specific capture of viable rare CTCs from artificial white blood cell samples. Furthermore, an in situ culture strategy has been proposed (Fig. 1). This work provides a promising strategy for viable isolation and purification of rare CTCs and it has great potential for achieving clinical validity.
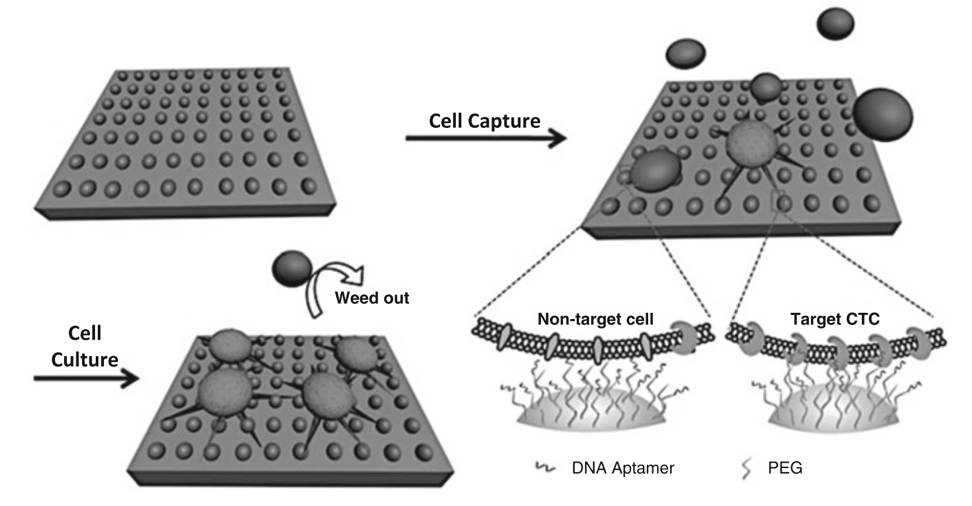 Figure 1. Scheme of the chitosan nanoparticle substrate for rare number CTC isolation from non-target cells followed by the optimized in situ culture of captured cells. (Sun, 2015)
Figure 1. Scheme of the chitosan nanoparticle substrate for rare number CTC isolation from non-target cells followed by the optimized in situ culture of captured cells. (Sun, 2015)
Aptamer-functionalized gold nanoparticles could be used to enrich and detect cancer cells using an aptamer-nanoparticle strip biosensor within a lateral flow device (Fig. 2). A pair of aptamers to Ramos cells – a thiolated aptamer (TD05) immobilized on gold nanoparticles and a biotinylated aptamer (TE02) immobilized in the test zone of a nitrocellulose membrane. The lateral flow strip device allowed cancer cells linked through TD05 aptamer with gold nanoparticles stay in the test zone. Accumulation of colloidal gold produced a visible red band. Unfortunately the large volumes of blood (more than 5 μl) masked the signal from the cancer cells because of the non-specific adsorption of erythrocytes on the membrane. The further optimization of this technology is required for the clinical implementation.
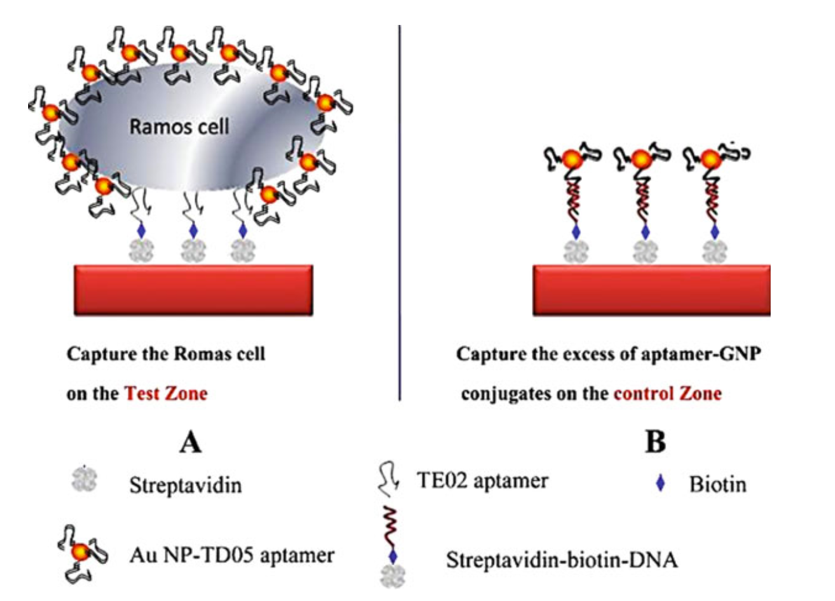 Figure 2. Scheme of the detection of Ramos cells on aptamer-nanoparticle strip biosensor (a) Capturing Au-NP-aptamer-Ramos cells on the test zone through specific aptamer-cell interactions (b) Capturing the excess of Au-NP-aptamer on the control zone through aptamer-DNA hybridization reaction. (Liu, 2009)
Figure 2. Scheme of the detection of Ramos cells on aptamer-nanoparticle strip biosensor (a) Capturing Au-NP-aptamer-Ramos cells on the test zone through specific aptamer-cell interactions (b) Capturing the excess of Au-NP-aptamer on the control zone through aptamer-DNA hybridization reaction. (Liu, 2009)
Technologies combining aptamer-functionalized nanoparticles with microfluidics can greatly enhance the robustness of aptamer-based CTC capture. A laminar flow flat channel microfluidic device allowed capture CCRF-CEM cells from blood due to the aptamer Sgc8 immobilized on gold nanoparticles. The problem of the cancer cell diversity could be solved by using aptamer-functionalized barcode particles to capture and detect various types of CTCs (Fig. 3). Variations in reflection properties of different spherical colloidal crystal clusters each modified with an aptamer to a certain CTC type, act as a code for analyses. Dendrimers were used to amplify the effect of the aptamers, allowing for increased sensitivity, reliability, and specificity in CTC capture, detection and release.
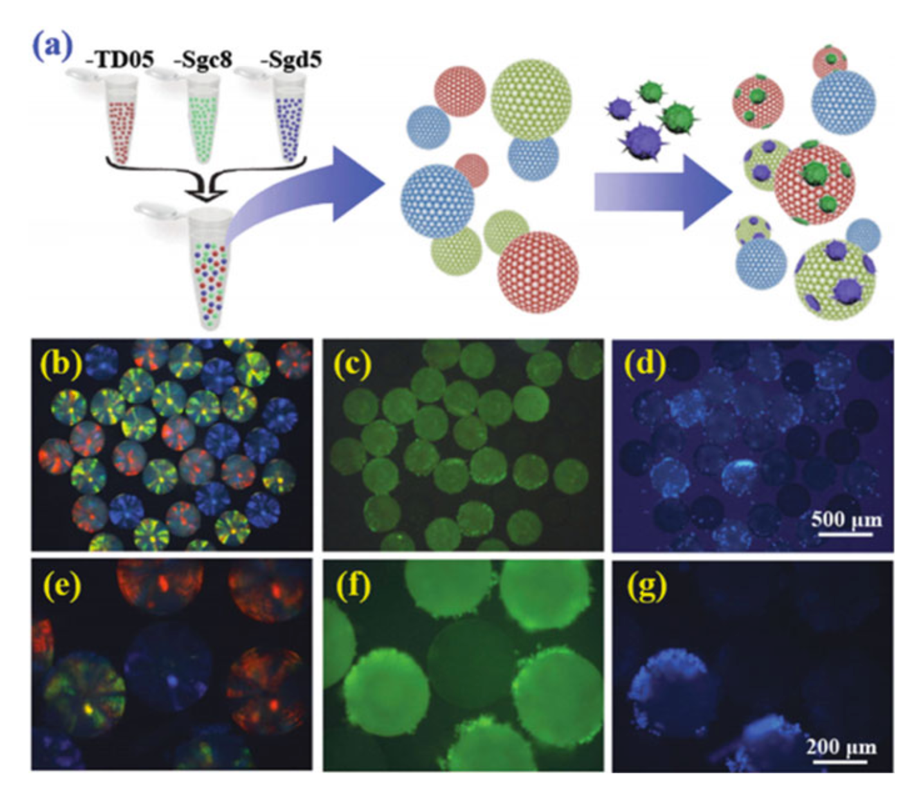 Figure 3. Scheme showing the barcode particles capturing multiple types of CTCs. Various aptamers, TD05, Sgc8, and Sgd5, were used; and green and blue-stained cells were used as the target cells (Zheng, 2014)
Figure 3. Scheme showing the barcode particles capturing multiple types of CTCs. Various aptamers, TD05, Sgc8, and Sgd5, were used; and green and blue-stained cells were used as the target cells (Zheng, 2014)
References
- Sun N.; et al. A cellular compatible chitosan nanoparticle surface for isolation and in situ culture of rare number CTCs. Small. 2015, 11(40):5444–5451.
- Zhao L.; et al. Enhanced and differential capture of circulating tumor cells from lung cancer patients by microfluidic assays using aptamer cocktail. Small. 2016, 12 (8):1072–1081.
- Liu G.; et al. Aptamer-nanoparticle strip biosensor for sensitive detection of cancer cells. Anal Chem. 2009, 81(24):10013–10018.
- Shen Q.; et al. Specific capture and release of circulating tumor cells using aptamer-modified nanosubstrates. Adv Mater. 2013, 25(16):2368–2373.
- Zheng F.; et al. Aptamer-functionalized barcode particles for the capture and detection of multiple types of circulating tumor cells. Adv Mater. 2014, 26(43):7333–7338.