Chromosome Painting Protocol of Mouse Chromosomes
GUIDELINE
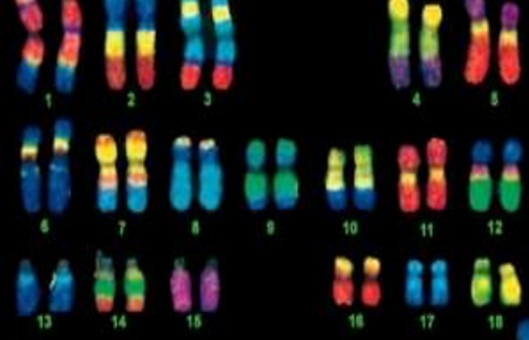
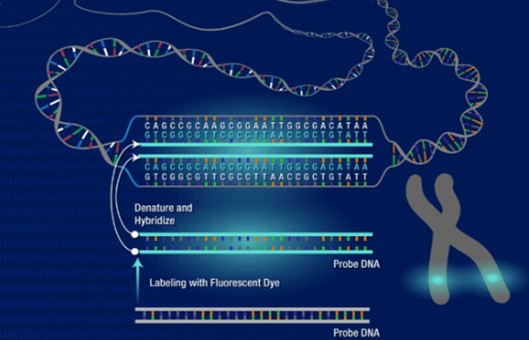
- Chromosome painting enables the visualization of chromosomes and has been used extensively in cytogenetics. Chromosome paint probes, which consist of a pooled composite of DNA-FISH probes, bind to nonrepetitive sequences for individual chromosomes.
- Here we describe the process of using chromosome painting to study the organization of chromosomes without fragmenting the nucleus. This method can be used to analyze chromosome position and identify translocations and ploidy within the nucleus. The preservation of nuclear morphology is crucial in understanding inter-chromosomal interactions and dynamics in the nucleus during the cell cycle.
METHODS
- Probe preparation. Heat-denature Whole Chromosome Paint in a 1.5 microcentrifuge tube in a heat block set at 80°C for 10 min, then cool to 37°C for 60 min until use in step 10.
- Rehydrate slides in 2×SSC for 10 min on ice, on a Belly Dancer, or a general platform rocker.
- Depurination slides with 0.1 N HCl for 5 min at RT to fragment DNA.
- Wash slides with cold 1×PBS for 2-5 min each, with rocking. Repeat the wash two additional times.
- Dehydrate slides in a cold ethanol series. 70%, 80%, and 100% for 2-5 min each on ice, with rocking.
- Pour out ethanol and air-dry slides in a Coplin jar for 1 min at RT.
- Place slides on a slide warmer until slides are dry (4 min).
- Emerge slides in prewarmed denaturation solution at 80°C for 7.5 min in a hybridization oven (temperature and time will vary and cell-type specific).
- Following heat denaturation, immediately dehydrate slides with a second series of ice-cold ethanol washes. 70%, 80%, and 100% for 2-5 min each on ice, while rocking.
- Pour out ethanol and air-dry slides in a Coplin jar for 1 min at RT.
- Place slides on a slide warmer until slides are dry (4 min).
- Apply 2.5 μL of the Whole Chromosome Paint mixture to the center of each 8 mm well on the slide, 2 wells per slide without any air bubbles. Immediately use a pair of forceps to carefully place a mini 8 mm coverslip over each well to seal the sample.
- Overlay a large coverslip over the mini 8 mm coverslips and seal the edges with 700 μL of rubber cement with a trimmed pipette tip.
- Place coverslipped slides at 80°C for 7.5 min in a hybridization oven (temperature and time will be cell-type dependent) to codenature both chromosome probe and sample.
- Place denatured slides in a humidified chamber and incubate at 37°C overnight.
- The following day, carefully remove both large/small coverslips by breaking the sealed edge of rubber cement with forceps. Slowly and carefully peel off, with the forceps tip slightly underneath the large coverslip edge, both coverslips simultaneously.
- Immediately following coverslip removal, submerge slides in a Coplin jar containing preheated post hybridization Wash buffer at 42 °C for 10 min in a hybridization oven, while rocking. Repeat two times.
- Incubate slides with a nuclear counterstain. DAPI or TO-PRO3 for 5 min at RT.
- Washed slides in a Coplin jar containing 1×PBS for 5 min at RT, while rocking.
- Use forceps to remove any trace amounts of rubber cement. Dry areas around the wells with Kim Wipes. Mount wells on the slide with Prolong Antifade and large coverslips.
- Seal coverslips with nail polish.
Creative Bioarray Relevant Recommendations
- Explore our Chromosome Painting Service, which is aimed at cell karyotype analysis, discovery and location of some non-random chromosome folding, comparative genomics research, identification of chromosome position in the nucleus, chromosome translocation, and ploidy changes, and so on. Our chromosome painting service provides karyotype and chromosome analysis for a single species, as well as comparison chromosome painting services for two species.
NOTES
- Multiple chromosome probes can be combined for a single application in the hybridization step.
- Depurination is an essential step to increase accessibility for chromosome paint probes to their target chromosome.
- Cut the bottom of a 1000 μL tip to pipette rubber cement as it is a viscous aqueous solution.
- Glass slides will absorb a large amount of heat of the denaturation solution immediately after submersion and thus decreases the temperature of the denaturation solution. To minimize this temperature change, slides must be prewarmed.
- If there are air bubbles or insufficient covering of the cells with chromosome paint, there will be areas devoid of staining when the slide is imaged.
- In situ, humidified chambers can be made by using pipette tip boxes or slide boxes that can be sealed/closed containing moistened paper towels in a 50% formamide, diluted in dd H2O, solution.
- The small coverslip will adhere to the large coverslip. If the small coverslip remains after the large coverslip is peeled off, carefully submerge the slide in wash solution a few times until it comes off, or remove with a coverslip with forceps.
RELATED PRODUCTS & SERVICES
Reference
- Hua LL, Mikawa T. (2018). "Chromosome Painting of Mouse Chromosomes." Methods Mol Biol. 1752), 133-143.