3D Multi-Cell-Type Liver Organoids of Non-alcoholic Fatty Liver Diseases
Toxicology In Vitro. 2024 Feb; 94: 105728.
Authors: Bronsard J, Savary C, Massart J, Viel R, Moutaux L, Catheline D, Rioux V, Clement B, Corlu A, Fromenty B, Ferron PJ.
INTRODUCTION
Primary human hepatocytes (pHHs) have been put forward as the "gold standard" model for predicting DILIs in vitro. Interestingly, pHHs exposed to free fatty acids (FAs) accumulate lipids and can be used as a model of steatosis. However, pHHs have some critical limitations, such as limited availability, the progressive loss of hepatic functions, and therefore incompatibility with chronic drug exposure. The development of in vitro models that recapitulate critical liver functions is essential for accurate assessments of drug toxicity.
METHODS
- Progenitor HepaRG cells were seeded at a density of 2 × 106 cells in a 75 cm2 flask in William's E culture medium (1×) supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 50 μM sodium hydrocortisone hemisuccinate, 5 μg/mL insulin, 50 U/mL penicillin, and 50 μg/mL streptomycin. Starting two weeks after seeding, the cells were cultured for two additional weeks in the same medium supplemented with 2% DMSO; this resulted in differentiation into both cholangiocyte- and hepatocyte-like cell populations. The peripheral blood mononuclear cells were isolated by differential centrifugation from buffy coat donations.
- The multi-cell-type liver organoids (HML) were obtained by seeding a solution of isolated cells in the micro-mold system. The mixed cells (80% differentiated-HepaRG cells, 10% macrophages, and 10% LX-2 cells) were seeded at a density of 2000 cells per organoid. The HML organoids were treated with free fatty acids (FAs) to induce steatosis. The mixture was composed of 150 μM stearic acid and 300 μM oleic acid and was prepared with FA-free BSA. Treatments were initiated on day (D) 5 after seeding and repeated every other day until D14.
- HML organoids were harvested, fixed in 5% formalin for 60 min, and washed in PBS. After the organoids had been embedded in paraffin, blocks were cut into 4 μm slices. The latter were mounted on positively charged slides and dried at 58°C for 60 min. Each slide was stained with hemalun-eosin-saffron (HES) reagent, to select the best slices before immunohistochemical staining.
- Browse our recommendations
| Product/Service Types | Description |
| Hepatic Cells | We offer 33 types of human hepatic cells including human liver carcinoma epithelial cells, human liver sinusoidal microvascular endothelial cells, human liver tumor-associated endothelial cells, human hepatic sinusoidal endothelial cells, etc. |
| Immunohistochemistry (IHC), Immunofluorescence (IF) Service | Creative Bioarray offers the best immunohistochemistry and immunofluorescence service. |
| Histological Stains & Dyes | Creative Bioarray provides a series of histological staining methods and related products to help our clients achieve the best dyeing purposes. |
| DNA/RNA Extraction Service | Creative Bioarray offers extraction services of DNA and RNA including large mRNA, microRNA, and siRNA from a wide range of starting materials with different scales/volumes for most applications or bespoke solutions. |
RESULTS
- HML organoids were harvested on D5, D7, D10, and D14 of culture and analyzed histologically. HES staining showed that the cells had a segregated organization in the organoid. The organoid's diameter ranged from 200 to 300 μm, and a necrotic area in the center of the organoid was never observed. CYP3A4 immunohistochemical staining showed that typical, large, polygonal hepatocytes were distributed throughout the organoids. The stellate cells (revealed by vimentin staining) self-organized over the 14-day culture period and spread over the outside of the organoids. The quantification of CD68-positive cells revealed that the M1 macrophages in the organoids remained differentiated throughout the culture period. These results were confirmed by quantitative image analysis of the CD68- and vimentin-positive cells.
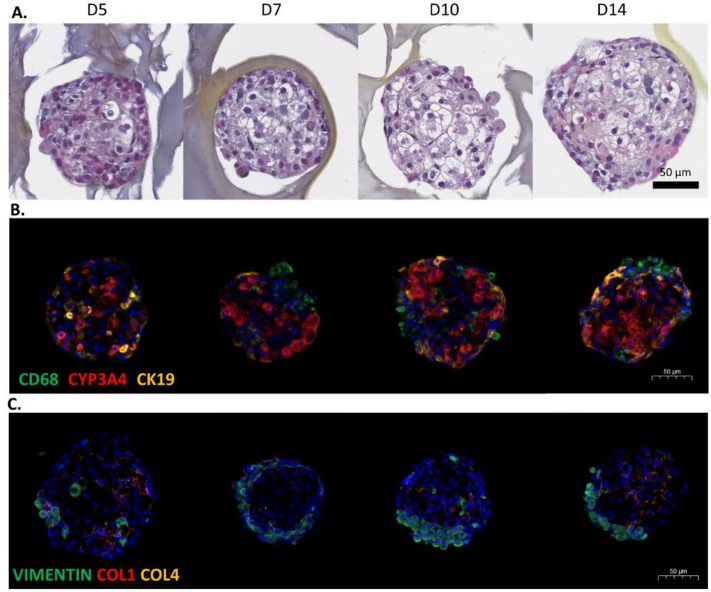 Fig. 1 Characterization of the HML organoid culture.
Fig. 1 Characterization of the HML organoid culture.
- The quantification of CYP3A4- and CK19-positive cells showed that the number of HepaRG cells within the organoid remained stable over the 14-day culture period. Hepatic markers hepatocyte nuclear factor-4 alpha (HNF4α) and albumin were slightly upregulated between D7 and D10 and between D5 and D7, respectively; expression remained high until D14. The expression levels of the phase 1 and 2 xenobiotic metabolizing enzymes CYP2E1, CYP3A4, and UGT1A1 increased over time. Stellate cells (as evidenced by A-SMA and COL1A1 labeling) and M1 macrophages (CD68) were maintained from D5 to D14.
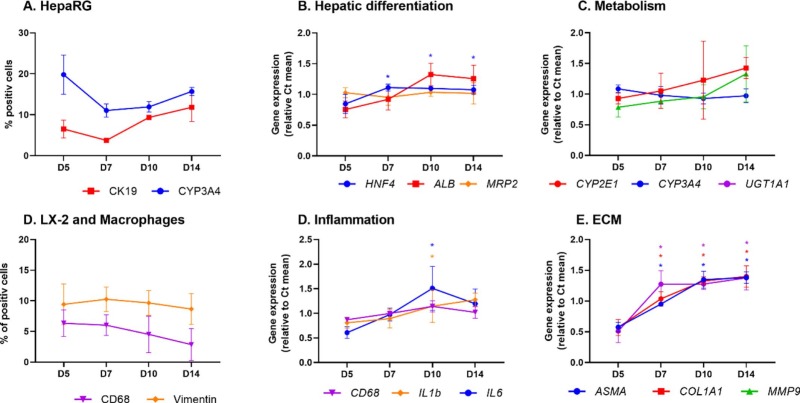 Fig. 2 Changes over time in the expression of cell markers in HML organoids cultured for 5 to 14 days, based on RT-qPCR assays of total RNA and image analysis.
Fig. 2 Changes over time in the expression of cell markers in HML organoids cultured for 5 to 14 days, based on RT-qPCR assays of total RNA and image analysis.
- Lipid labeling showed that exposure to the FA mixture was associated with an increase in the number of neutral lipid droplets in the treated organoids after 9 days of exposure. Organoids exposed to FA for 9 days presented several characteristics of non-alcoholic fatty liver disease (NAFLD). The FA treatment was associated with greater production of COL1 and COL4 in the organoids, together with fibrosis. The level of CYP3A4 gene expression was significantly lower in FA-treated HML organoids, and that of COL1A1 was significantly higher. The expression of lipid-metabolism-related genes (such as lipid droplet coating protein perilipin 2 (PLIN2) and apolipoprotein B (APOB) gene) and levels of protein secretion were significantly higher in FA-exposed HML organoids.
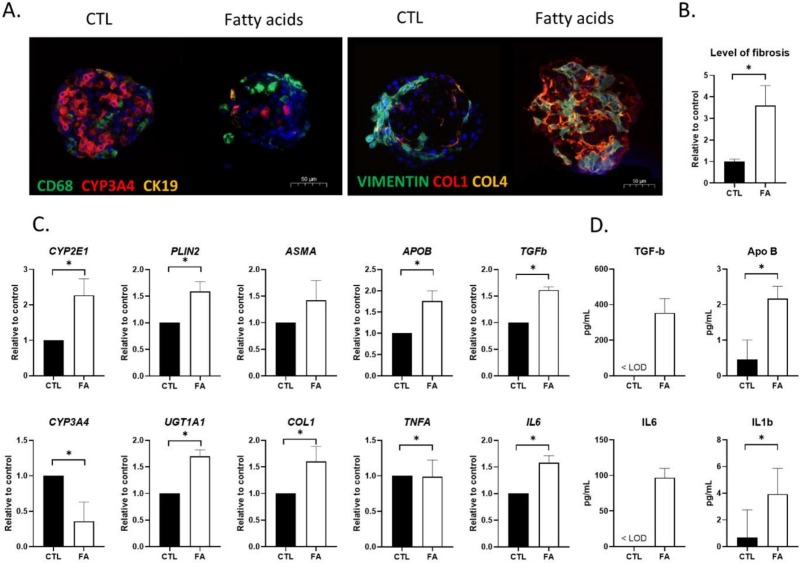 Fig. 3 Characteristics of NAFLD induced by the exposure of HML organoids to FAs.
Fig. 3 Characteristics of NAFLD induced by the exposure of HML organoids to FAs.
SUMMARY
Three-dimensional multi-cell-type liver organoids (hereafter "HML organoids") were generated from HepaRG cells, primary human macrophages, and hepatic-stellate-cell-derived LX-2 cells. We also developed an NAFLD model by culturing HML organoids for 9 days with a mixture of stearic and oleic acids. The exposed organoids showed typical features of steatosis and expressed fibrosis markers.
RELATED PRODUCTS & SERVICES
Reference
- Bronsard J, et al. (2024). "3D multi-cell-type liver organoids: A new model of non-alcoholic fatty liver disease for drug safety assessments." Toxicol In Vitro. 94: 105728.