What Is Stem Cell-Derived Exosome Therapy?
Signal Transduction and Targeted Therapy. 2024 Jan 12; 9 (1): 17.
Authors: Tan F, Li X, Wang Z, Li J, Shahzad K, Zheng J.
INTRODUCTION
Stem cell-based therapy, as a modality of regenerative medicine, has generated tremendous attention, as it offers new options for patients suffering from previously incurable diseases. Subsequently, thousands of related clinical trials have been registered, covering a wide spectrum of medical problems, such as musculoskeletal and neurological disorders, immune diseases, hematological dysfunctions, and degenerative conditions. However, some trials have failed to show any benefit in the clinic. This is likely due to the inevitable limitations of stem cell therapy, such as infusion toxicity, immunogenicity, tumorigenic potentials, and ethical issues. Exosomes, secreted by almost all cell types including stem cells, have been posited as a safer and more versatile alternative to stem cell therapy.
Exosomes are nanoscale, spherical, and lipid bi-layered single membrane extracellular vesicles, which act as intercellular messengers. Exosomes have been regarded as miniature versions of their parental cells, partially because exosomes from a certain cell type provide cell-specific or unique sets of biomolecules. In addition, stem cells have been found to function in a paracrine fashion through their soluble secretome including exosomes. In other words, stem cell-derived exosomes (SC-Exo) inherit similar therapeutic effects from their parental cell of origin, e.g., anti-inflammation, immunomodulation, and tissue regeneration. Collectively, stem cell-derived exosomes are a potent surrogate for stem cell therapy without exhibiting the disadvantages their cellular counterparts present.
Table 1. The comparison between stem cell therapy and stem cell-derived exosome therapy.
| Treatment modality | Advantages | Limitations |
| Stem cell therapy | Multilineage differentiation potential; applicable to the treatment of a wide range of diseases; extensive accumulation of laboratory and clinical data; easy to isolate and possible for mass-production; well-developed regulatory guidelines. | Short-lived viability and low engraftment after injection; stringent storage and transport requirements; tumorigenic potential; infusion toxicity; immunogenicity; ethical issues. |
| Stem cell-derived exosome therapy | Comparable therapeutic effects to stem cells but much smaller; more concentrated functional cargos; modifiable at its surface and in its cargos; versatile delivery modalities; stable for long-term storage and transport; negligible risk of tumorigenesis and immune response; lack of ethical issues. | Batch-to-batch inconsistency; no standardized protocol for purification and storage; relatively low yield for large-scale manufacturing; no industry-standard quality specifications; insufficient regulatory control. |
General Background of Exosome Therapy
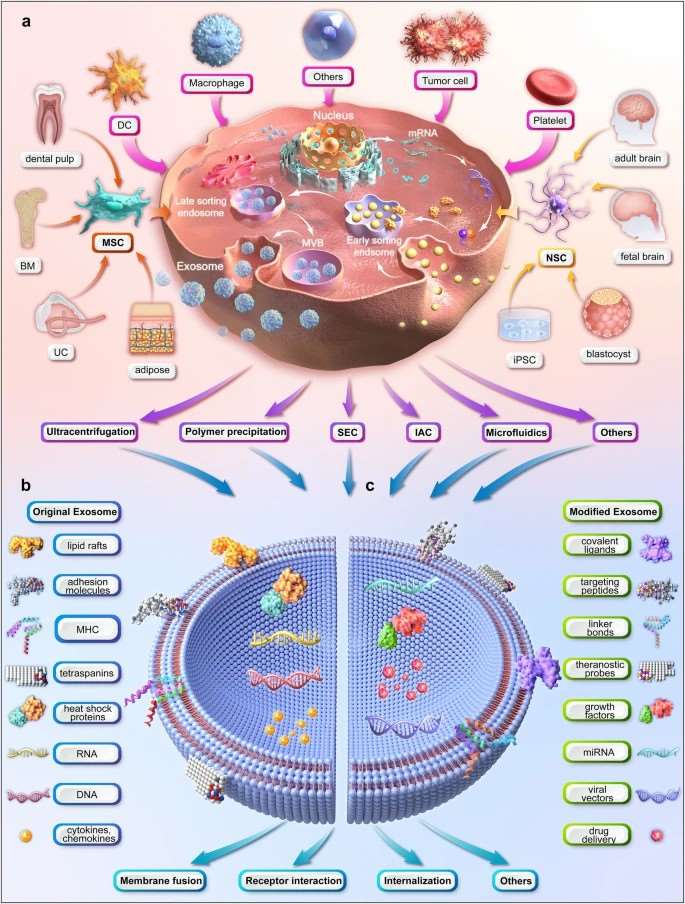 Fig. 1 Illustration of the upstream measures of exosome therapy.
Fig. 1 Illustration of the upstream measures of exosome therapy.
- Biogenesis, composition, and uptake of exosomes. Exosomes differ from other types of primary extracellular vesicles (e.g., apoptotic bodies and microvesicles) in terms of size, content, and production mechanism. The most popularly accepted mechanism of exosome formation, i.e., an endosomal route. Exosomes contain numerous molecules, including proteins, glycoconjugates, lipids, nucleic acids, metabolites, and other bioactive substances. There are various uptake mechanisms once exosomes reach the recipient cell, all of which can be categorized into membrane fusion, receptor interaction, and internalization. Finally, the exosomal cargos are released into the cytoplasm, the process of which depends on the source of the exosome, the nature of the cargo, and the metabolic state of the recipient cell.
- Production, isolation, and purification of exosomes. There are various methods of upscaling exosome production, which is categorized into biochemical strategies (e.g., LPS, BMP-2, HIF-1α, and IFN-γ and TNF-α), physical strategies (hypoxia, thermal stress, and starvation), mechanical strategies (shear stress and 3D culturing) and instrumental strategies (hollow-fiber bioreactors and stirred tank bioreactors). The currently available techniques for exosome isolation and purification are based on their size, surface charge, or immunoaffinity.
- Modification of exosomes. The modification of exosomes is classified into internal strategies (e.g., drug loading) and external strategies (e.g., surface modification). On the one hand, exosomes may be an ideal therapeutic carrier to deliver drugs, nucleic acids, and vaccines due to their advantages in stability, non-immunogenicity, and targeting of recipient cells. On the other hand, surface modification of exosomes is exemplified by genetic engineering of exosomal membrane or parental cells, chemical connection of targeting ligands, electrostatic interaction, and magnetic nanoparticle technology.
- Characterization and verification of exosomes. Size-oriented verification includes nanoparticle tracking analysis (NTA), dynamic light scattering (DLS), and tunable resistive pulse sensing (TRPS), whereas morphology-oriented analysis includes scanning electron microscopy (SEM) and transmission electron microscopy (TEM). In addition, cargo profiling is further subdivided into proteomic, lipidomic, and genomic analyses including western blotting, ELISA, flow cytometry, mass spectroscopy, and PCR.
Applications of Stem Cell-Derived Exosomes
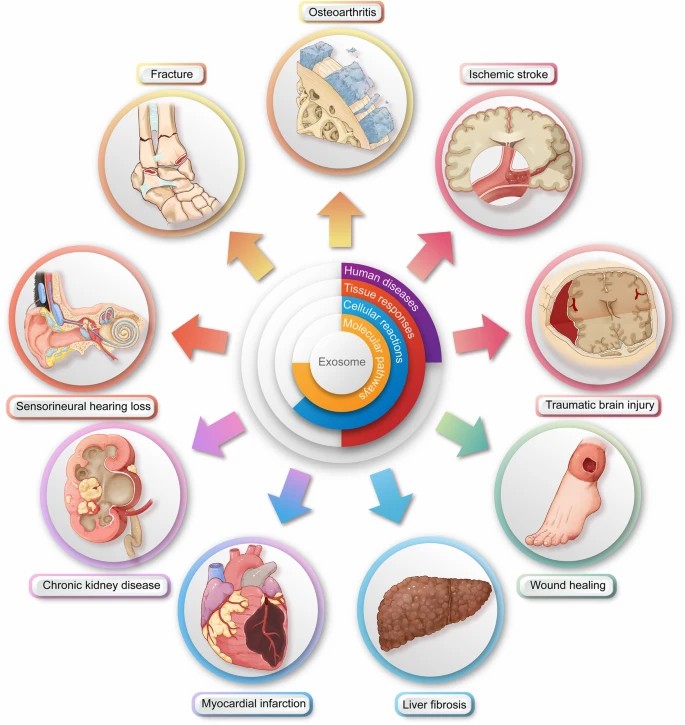 Fig. 2 Illustration of the downstream surgical applications of exosome therapy.
Fig. 2 Illustration of the downstream surgical applications of exosome therapy.
- SC-Exo therapy in fractures. As a promising alternative, exosome therapy for fracture healing mostly utilizes bone marrow-derived MSCs as a cellular supplier. Firstly, the progression of bone repair needs a variety of cells, e.g., inflammatory cells in the inflammation stage, endothelial and mesenchymal progenitor cells in the fibrovascular stage, osteoblasts and chondrocytes during bone formation, and osteoclasts during callus remodeling. Secondly, most of these cells can uptake exosomes, especially osteoblasts and vascular endothelial cells, which are most related to fracture healing. Lastly, upon exosome absorption, the gene expression of the recipient cells is modified, thereby activating various signaling pathways, causing various cellular and tissue responses and ultimately leading to improved fracture healing.
- SC-Exo therapy in strokes. Some groups have targeted neuroprotection and neurogenesis. Firstly, SC-exo therapy could inhibit neuronal cell death. Secondly, SC-exo therapy could protect cells of the central nervous system (CNS). Thirdly, SC-exo therapy could improve post-stroke neurogenesis. Some groups have targeted the inhibition of the neuroinflammation. Firstly, unmodified SC-exo therapy exhibited an anti-inflammatory effect through exosomal miRNAs. Secondly, the anti-inflammatory effect of SC-exo therapy could be enhanced by modifying the exosomes.
- SC-Exo therapy in plastic surgery. More than half of relevant work using SC-exo therapy to boost cutaneous wound healing is MSC-based. In addition to wound healing, other plastic surgery-related diseases have been proven to be suitable targets for SC-exo therapy. These include but are not limited to 1, skin grafting, such as skin flaps; 2, tissue loss, such as craniofacial defect; 3, autoimmune skin diseases, such as scleroderma; 4, skin infections, such as leishmaniasis; 5, hair transplantation, such as for alopecia; 6, skin aging.
Creative Bioarray Relevant Recommendations
| Product/Service Types | Description |
| Mesenchymal Stem Cells | Creative Bioarray offers a broad range of mesenchymal stem cell products including cryopreserved human & animal MSCs from various tissue sources, optimized media for expansion and cryopreservation, kits and media to differentiate the cells into adipocytes and osteocytes, and characterization antibodies and kits. |
| Exosome Isolation Tools | Creative Bioarray aims to develop the best quality exosome isolation tools with optimized conditions to help our customers obtain pure exosomes with a higher yield. |
| Exosome Identification | Creative Bioarray's exosome identification service makes it easy to detect and characterize exosomes with our experience working on exosomes. |
RELATED PRODUCTS & SERVICES
Reference
- Tan F, et al. (2024). "Clinical applications of stem cell-derived exosomes." Signal Transduct Target Ther. 9 (1): 17.