SYK Coordinates Neuroprotective Microglial Responses
Cell. 2022 Oct 27; 185(22): 4135-4152
Authors: Ennerfelt H, Frost EL, Shapiro DA, Holliday C, Zengeler KE, Voithofer G, Bolte AC, Lammert CR, Kulas JA, Ulland TK, Lukens JR.
INTRODUCTION
- Neurodegenerative disorders, such as Alzheimer's disease (AD), are major public health issues that are likely to increase in prevalence with the aging population.
- Recent studies have begun to reveal critical roles for the brain’s professional phagocytes, microglia, and their receptors in the control of neurotoxic amyloid beta (Aβ) and myelin debris accumulation in neurodegenerative disease. However, the critical intracellular molecules that orchestrate neuroprotective functions of microglia remain poorly understood.
- Spleen tyrosine kinase (SYK)is perhaps best known for the critical role that it plays in mounting protective antifungal immune responses downstream of C-type lectin (CLEC) receptors expressed on innate immune cells. In addition, SYK has been identified as the central kinase that instructs signaling and effector functions downstream of TREM2, CD33, and CD22 receptors. The activation of SYK in microglia surrounding Aβ and other forms of neurotoxic material spurred our interest in delineating whether SYK is a critical regulator of microglial responses in neurodegenerative disease.
METHODS
- At first, the researchers generated Sykfl/fl Cx3cr1ERT2Cre mice (hereafter referred to as SykΔMG mice) as a genetic tool to delete SYK from microglia, to investigate how SYK signaling in microglia impacts Aβ-mediated neurodegenerative disease. Then, they crossed SykΔMG mice with 5xFAD mice, an AD mouse model that develops aggressive Aβ pathology starting at 1.5 months of age. 5xFAD SykΔMG mice were given tamoxifen food for 2 weeks after weaning to induce deletion of SYK.
- Using experimental techniques such as brain sample preparation, ELSA and immunofluorescence microscopy, the researchers evaluated the neuronal health of the 5xFAD and 5xFAD SykΔMG mice. To better understand how Syk deficiency in microglia impacts brain function, they evaluated the performance of 4-month-old 5xFAD and 5xFAD SykΔMG mice in the Morris water maze (MWM) and elevated plus maze (EPM).
- To ascertain whether SYK signaling also impacts microglia activation in response to Aβ, the study first evaluated differences in microglia morphology via Sholl analysis. To elucidate whether SYK affects DAM acquisition in response to Aβ-driven neuropathology, they performed bulk RNA sequencing (RNA-seq) on magnetic bead-sorted CD11b+ cells isolated from the brains of 5-month-old Sykcon, SykΔMG, 5xFAD, and 5xFAD SykΔMG mice.
- We measured Aβ engulfment by microglia using immunofluorescence staining. Then, we explored in a secondary experimental system, 5-month-old 5xFAD SykΔMG mice and 5xFAD littermate controls received an intraperitoneal (i.p.) injection of Methoxy-X04, a brain penetrant dye that labels fibrillar Aβ. After 3 h, we harvested brains from these mice and used flow cytometry to quantify the percentage of microglia that had taken up Methoxy-X04-labeled Aβ.
RESULTS
- SYK-deletion in 5xFAD SykΔMG mice leads to significantly elevated accumulation of Aβ in the cortex, hippocampus, and thalamus at 5 months of age; Aβ plaques in the cortex and hippocampus of 5xFAD SykΔMG mice exhibited lower sphericity than the plaques found in 5xFAD littermate controls. Altogether, these findings indicate that SYK is critical for Aβ consolidation and plaque compaction by microglia in 5xFAD mice.
- Compared with 5xFAD controls, 5-month-old 5xFAD SykΔMG mice had a 1.5-fold increase in plaque-associated dystrophic neurites in the cortex, accumulated heightened hyperphosphorylated tau labeled with AT8 antibody. In the MWM test, it took markedly longer for 5xFAD SykΔMG mice to find the hidden platform in comparison to 5xFAD littermate controls; similarly, 5xFAD SykΔMG mice spent more time exploring the open arms of the maze in the test of EPM. Taken together, these data demonstrate a critical role for microglial SYK in neuronal health and memory impairment.
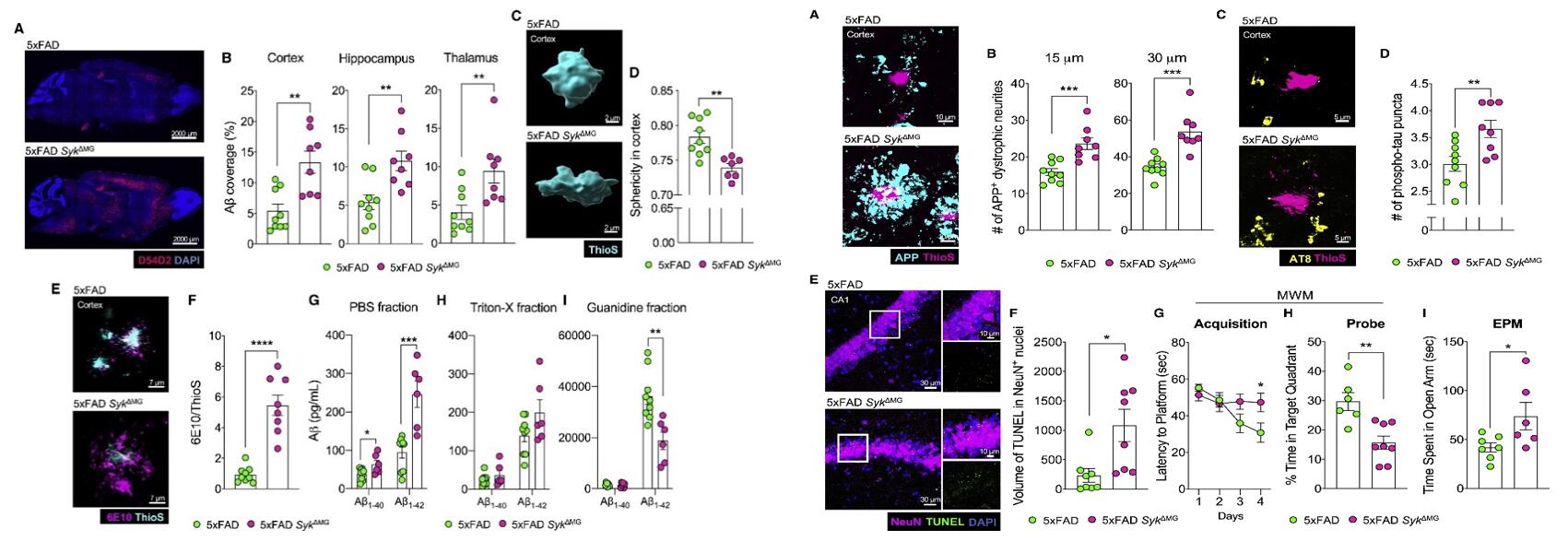 Fig. 1 Left: Deletion of Syk in microglia leads to increased Aβ burden and altered plaque composition in 5xFAD mice; Right: Loss of Syk in microglia negatively affects neuronal health and exacerbates AD-related behaviors in 5xFAD mice.
Fig. 1 Left: Deletion of Syk in microglia leads to increased Aβ burden and altered plaque composition in 5xFAD mice; Right: Loss of Syk in microglia negatively affects neuronal health and exacerbates AD-related behaviors in 5xFAD mice.
- Compared with 5xFAD controls,non-plaque-associated microglia in the cortex and hippocampus of 5xFAD SykΔMG mice had significantly more ramified processes. In contrast, resting morphological differences were not seen, suggesting that SYK-dependent morphological differences in microglia are specific to Aβ-driven neurological disease. 2,769 downregulated and 2,668 upregulated genes when comparing microglia isolated from 5xFAD SykΔMG and 5xFAD mice, and KEGG analysis revealed that many of the repressed genes in 5xFAD SykΔMG microglia were related to neurodegeneration. These findings suggest that SYK acts as a critical regulator of the transcriptional shift that microglia undergo in response to Aβ-associated neuropathology.
- 5xFAD microglia engulfed more than twice the amount of Aβ than 5xFAD SykΔMG microglia. Similarly, approximately twice the volume of Aβ was engulfed within CD68. Further study showed approximately 20% of 5xFAD microglia had ingested Aβ (Methoxy-X04+) while almost none of the 5xFAD SykΔMG microglia contained Methoxy-X04-stained Aβ. In total, these combined results provide evidence that SYK promotes the phagocytic capacity of microglia in response to Aβ.
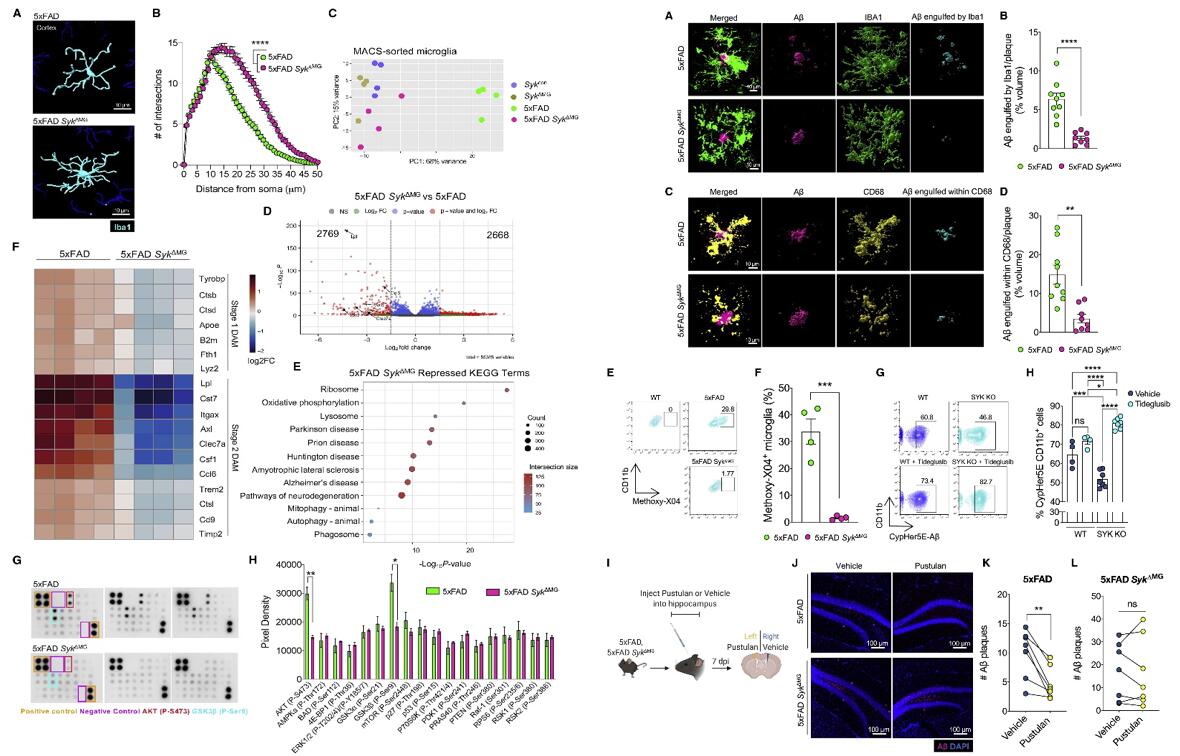 Fig. 2 Left: Defective activation of Syk-deficient microglia in 5xFAD mice; Right: SYK is critical for microglial uptake and phagocytosis of Aβ.
Fig. 2 Left: Defective activation of Syk-deficient microglia in 5xFAD mice; Right: SYK is critical for microglial uptake and phagocytosis of Aβ.
SUMMARY
- In this studies, targeted deletion of SYK in microglia leads to exacerbated Aβ deposition, aggravated neuropathology, and cognitive defects in the 5xFAD mouse model of Alzheimer's disease.
- Disruption of SYK signaling in this AD model was further shown to impede the development of disease-associated microglia (DAM), alter AKT/GSK3β-signaling, and restrict Aβ phagocytosis by microglia. Conversely, receptor-mediated activation of SYK limits Aβ load.
- SYK critically regulates microglial phagocytosis and DAM acquisition in demyelinating disease.
RELATED PRODUCTS & SERVICES
Reference
- Ennerfelt H, et al. (2022). "SYK coordinates neuroprotective microglial responses in neurodegenerative disease." Cell. 185 (22), 4135-4152.