Reprogramming of Neutrophils by Lung Mesenchymal Cells
Science Immunology. 2023 Feb 24; 8 (80): eadd5204.
Authors: Gong Z, Li Q, Shi J, Li P, Hua L, Shultz LD, Ren G.
INTRODUCTION
Neutrophils, the most abundant innate immune cells, function as crucial regulators of the adaptive immune system in diverse pathological conditions, including metastatic cancer. However, it remains largely unknown whether their immunomodulatory functions are intrinsic or acquired within the pathological tissue environment.
METHODS
- To determine whether the immunosuppression exerted by neutrophils is intrinsic or elicited by local environments of metastatic organs, we compared neutrophils residing in the hematopoietic site (i.e., BM), those circulating in the peripheral blood (PB), and those infiltrating the metastatic organ (lung) at the premetastatic stage. To test whether the lung environment serves to reprogram infiltrated neutrophils to acquire an immunosuppressive phenotype, we intravenously injected fluorescent dye–labeled BM-derived healthy neutrophils into naïve recipient mice.
- We first profiled BM, PB, and lung neutrophils derived from naïve (C57BL/6J), AT3 tumor–bearing, and AT3-gcsf orthotopic tumor–bearing mice using scRNA-seq. Using a modified experimental lung metastasis model, in which luciferase-labeled tumor cells were intravenously injected into orthotopic tumor-bearing mice.
- We cocultured mouse BM-derived neutrophils ex vivo with various types of freshly isolated lung stromal cells from naïve mice. Through RNA-seq, we next attempted to gain a broader understanding of how lung MCs modulate neutrophils at the transcriptional level. Through ingenuity pathway analysis (IPA) of the RNA-seq data from lung neutrophils versus PB neutrophils or lung MC-educated neutrophils versus control neutrophils.
- To better understand the in vivo role of the PGE2-dependent lung MC program in reprogramming neutrophils, we generated MC-targeted Ptgs2 conditional knockout (cKO) mice (Pdgfra-Cre; Ptgs2flox/flox or Ptgs2ΔMCs). We then determined how Ptgs2/PGE2 deficiency in MCs alters the immunosuppressive capacity of lung neutrophils in vivo. Next, we attempted to pharmacologically block the PTGS2 (prostaglandin-endoperoxide synthase 2)/PGE2 signaling pathway as an endeavor to develop clinically applicable approaches to treat lung metastasis.
RESULTS
- Neutrophils (CD45+CD11b+Ly6ClowLy6G+) isolated from premetastatic lungs had a more robust ability to suppress CD4+ and CD8+ T cell proliferation. Lung neutrophils were found to express higher levels of immunosuppression-associated genes. Exogenous eutrophils infiltrating the lung, substantially increased their expression of immunosuppression-associated genes, indicating a lung-specific capacity to reprogram infiltrated neutrophils. A rapid acquisition of the immunosuppressive phenotype by lung-infiltrating neutrophils as early as 4 hours after transfer.
- Lung neutrophils displayed a tissue-specific clustering distribution across the three different host conditions. Neither tumor-bearing nor host inflammation status notably altered the tissue-specific immunosuppressive gene expression pattern of neutrophils. These genes underwent a further significant up-regulation in the AT3-gcsf model. Such a changing pattern of lung neutrophils at the transcriptional level was reflected in their functional changes in suppressing T cells and NK cells. The strong host inflammation condition was superior to the weak one in accommodating colonization of AT3-Luc cells. In contrast, primary tumor growth did not significantly differ under these two host conditions.
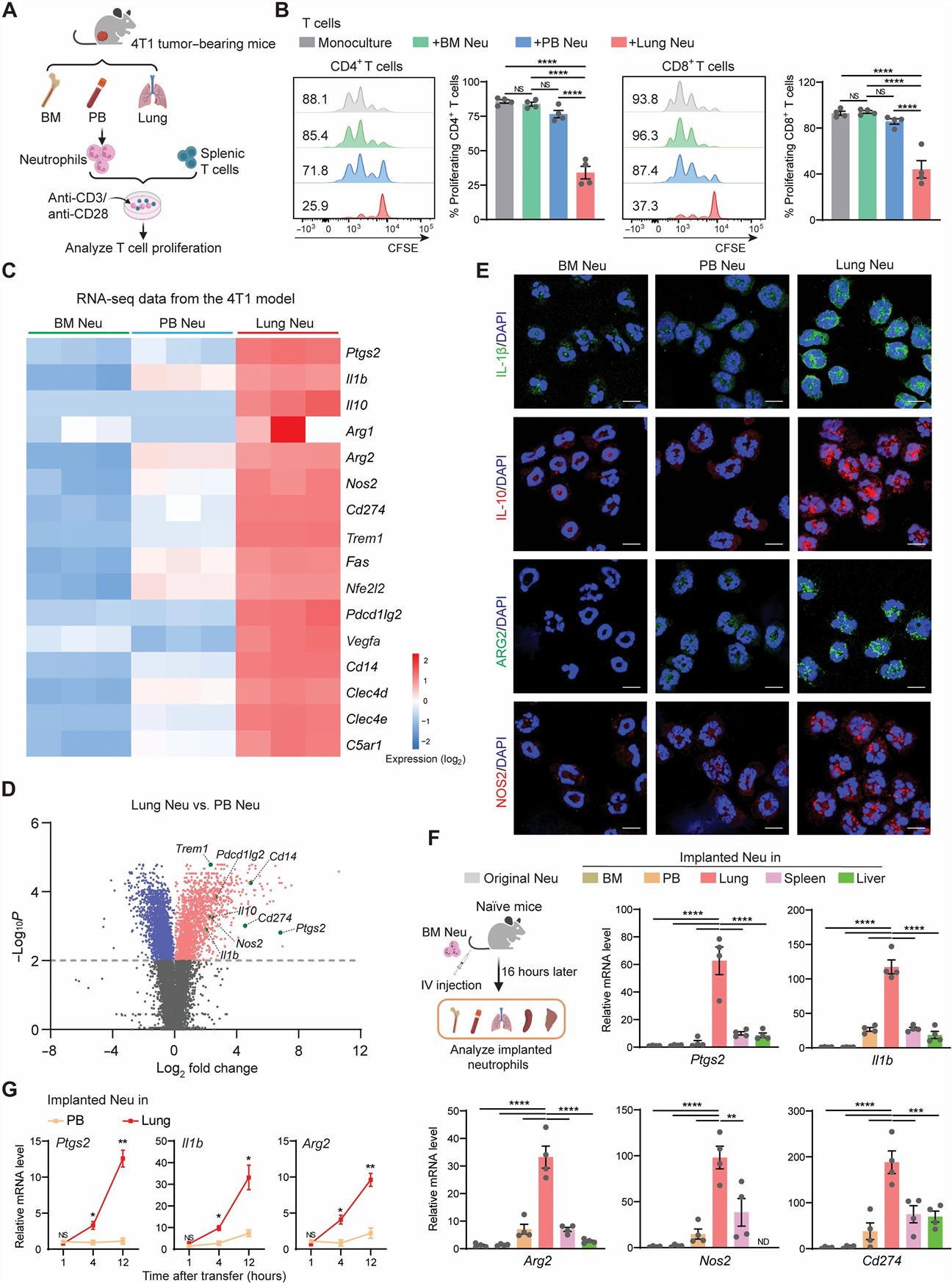
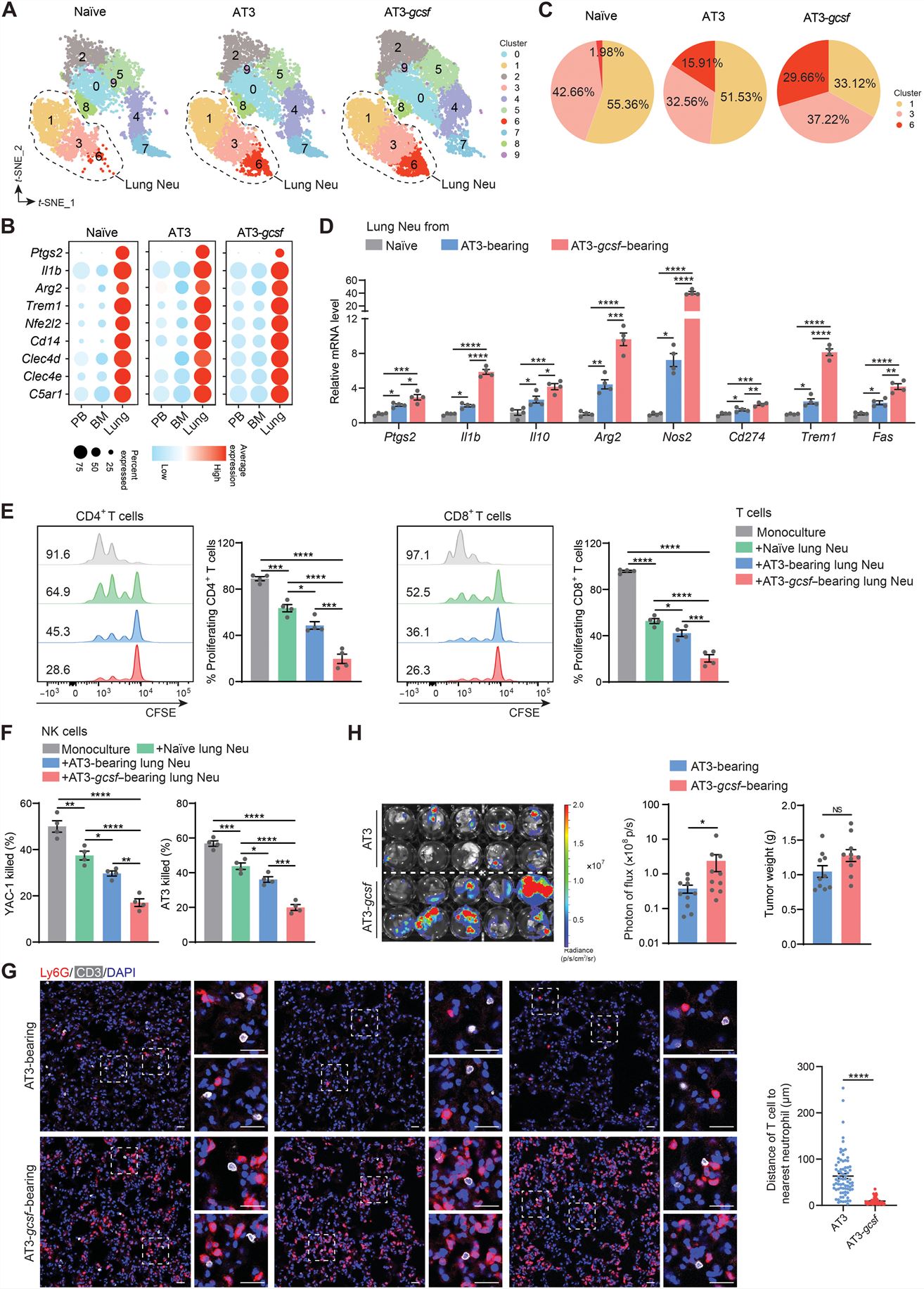
Fig. 1 Left: The immunosuppressive capacity of neutrophils is tissue dependent under both steady-state and tumor-bearing conditions; Right: Host inflammation strengthens the immunosuppressive capacity of lung neutrophils.
- Among the examined lung tissue cells, CD140a+ lung mesenchymal cells (MCs) significantly increased the neutrophil expression of these genes. Lung MCs was found to be superior to CD140a+ MCs isolated from other tissues in their ability to induce the immunosuppression-associated gene expression in neutrophils ex vivo. MCs were identified as the stromal component in the lung that reprograms neutrophils at the steady state. Lung MCs serves to reprogram neutrophils at the transcriptional and functional levels at the steady state, likely through soluble factors.
- Through an in vitro screen of these factors, PGE2, an inflammatory lipid mediator, was superior in up-regulating immunosuppression-associated genes in BM neutrophils. Further ex vivo experiments showed that IL-1β and IL-6 were capable of enhancing the effects of PGE2 in up-regulating neutrophil immunosuppression-associated genes. A combination of PGE2, IL-1β, and IL-6 acted similarly to lung MCs in immunosuppressive reprogramming of neutrophils at the transcriptional level and functional level.
- Lung neutrophils isolated from Ptgs2ΔMCs mice underwent a significant reduction in their suppression of T cell proliferation and NK cell cytotoxicity compared with their WT counterparts. In the ex vivo MC-neutrophil coculture system, dual inhibition of EP2 and EP4 showed a superior effect in diminishing lung MC–induced immunosuppression-associated gene expression in neutrophils compared with blockade of either receptor alone or blockade of IL-1β or IL-6. Elevated host inflammation, characterized by the increased immunosuppressive capacity of lung neutrophils, blunted antitumor immunity in the lung and was associated with loss of therapeutic efficacy of adoptive T cell–based immunotherapy in treating lung metastases.
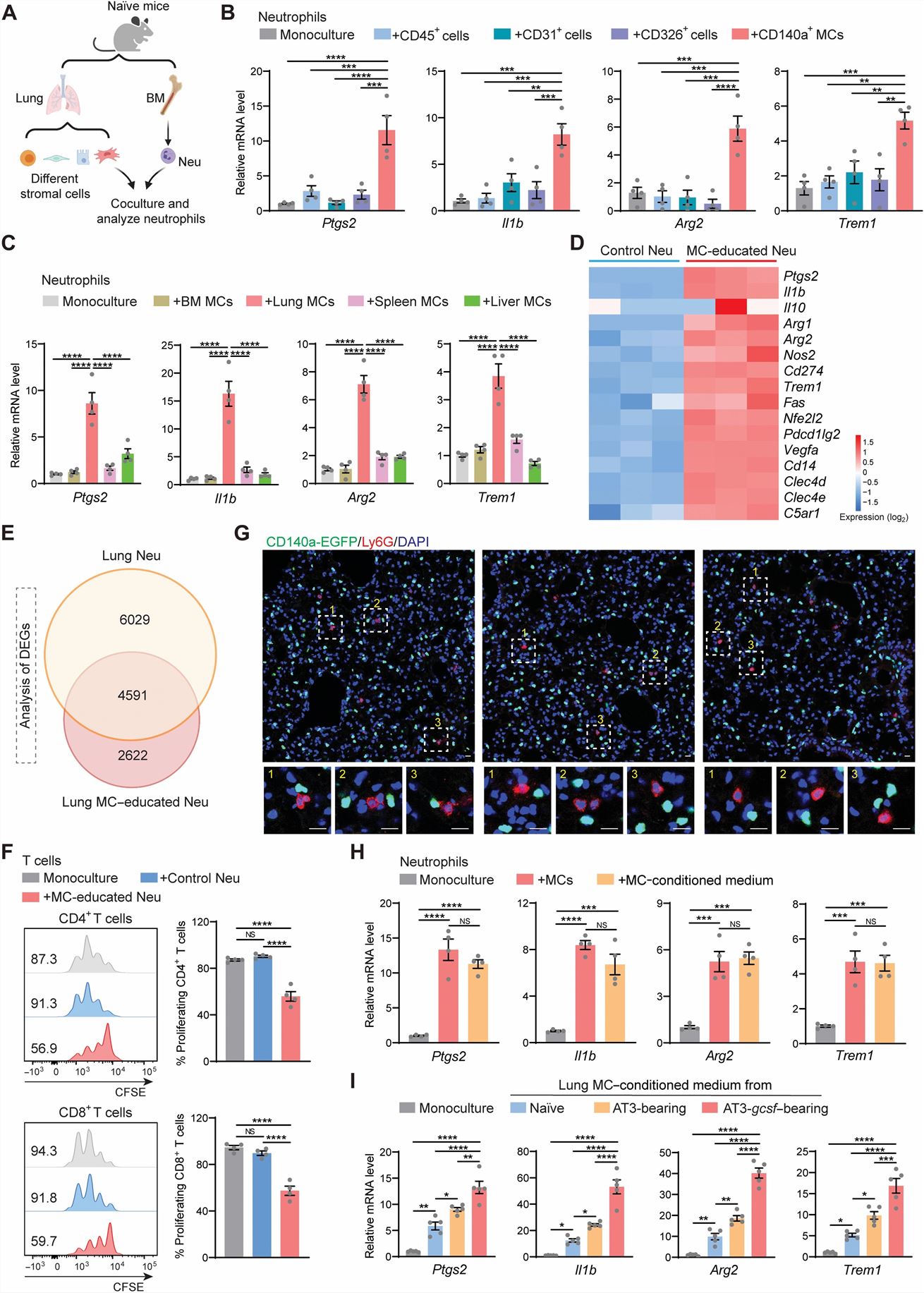
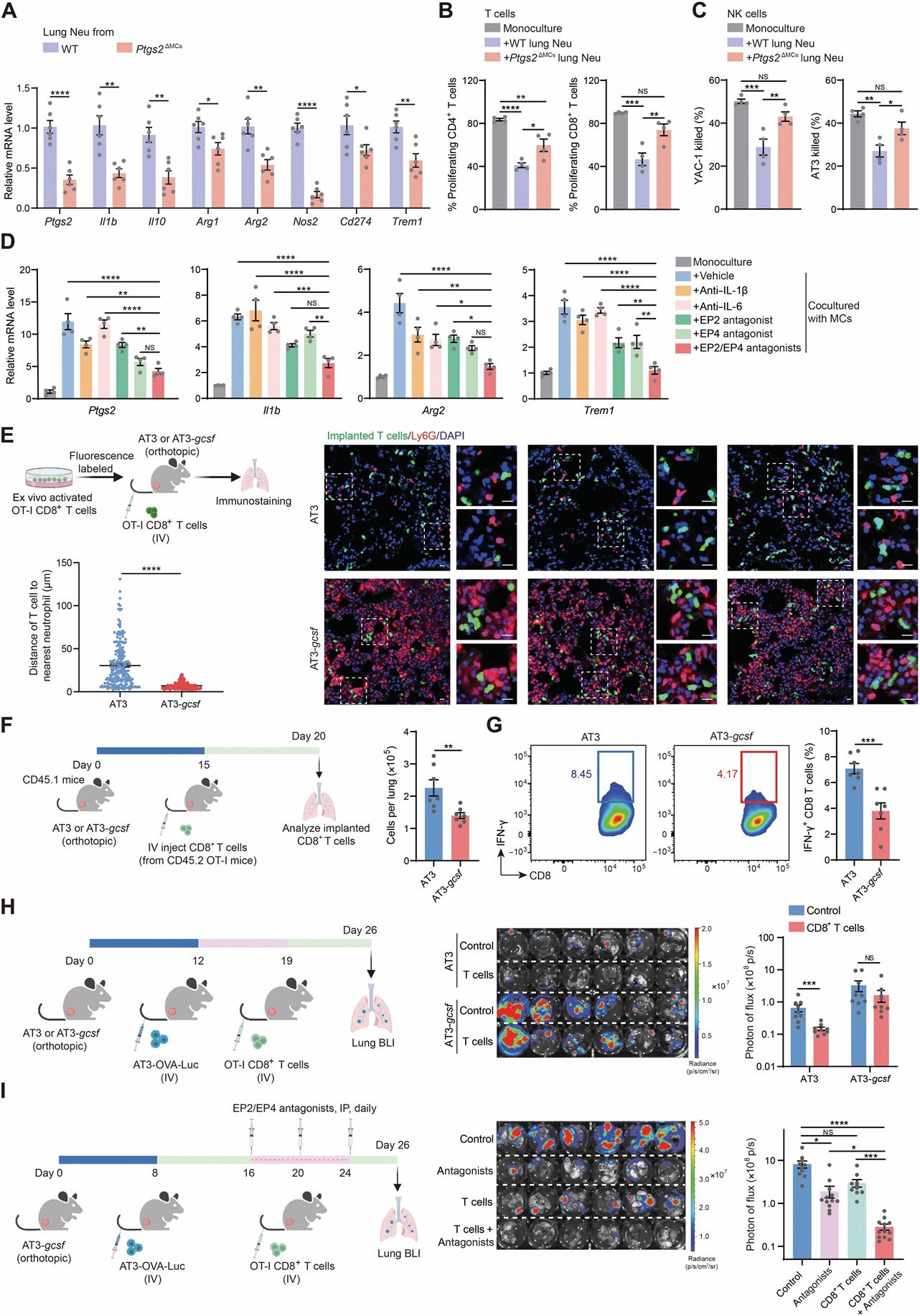
Fig. 2 Left: Lung MCs reprogram neutrophils to be immunosuppressive; Right: Targeting PGE2 signaling reduces the immunosuppressive capacity of lung neutrophils and improves the therapeutic efficacy of adoptive T cell-based immunotherapy in treating lung metastasis.
SUMMARY
- Using mouse models of lung-metastasizing breast cancer, we found that the lung stroma endows infiltrating neutrophils with immunosuppressive features enabling strong suppression of antitumoral T and natural killer cells, an effect further strengthened by host inflammation.
- Collectively, our results reveal that the immunoregulatory effects of neutrophils are induced by tissue-resident stroma and that targeting tissue-specific stromal factors represents an effective approach to boost tissue-resident immunity against metastatic disease.
RELATED PRODUCTS & SERVICES
Reference
- Gong Z, et al. (2023). "Immunosuppressive reprogramming of neutrophils by lung mesenchymal cells promotes breast cancer metastasis." Sci Immunol. 8 (80), eadd5204.