Inactive Disease in Lupus Patients Normalizes Ifnα and B-Cell Subsets
Cell Reports Medicine. 2023 Jan 17; 4 (1): 100894
Authors: Bradford HF, Haljasmägi L, Menon M, McDonnell TCR, Särekannu K, Vanker M, Peterson P, Wincup C, Abida R, Gonzalez RF, Bondet V, Duffy D, Isenberg DA, Kisand K, Mauri C.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease affecting multiple organ systems. Abnormal B cell proportions including expansion of atypical memory, also known as double-negative (DN) B cells, and autoantibody-secreting plasma cells contribute to autoimmune inflammation and tissue injury. We and others have previously shown that a finely tuned IFNα response is required to induce the differentiation of immature B cells into plasma cells that produce antibodies.
METHODS
- To evaluate whether patients with SLE develop endogenous autoantibodies to IFNα or/and other cytokines, we tested sera from 474 patients with SLE and 312 healthy controls (controls) for autoantibodies (auto Abs) against cytokines with the luciferase immunoprecipitation system (LIPS) assay.
- To understand the stability of the anti-IFN-Abs, we assessed the kinetics of anti-IFN-Ab production, circulating IFNα levels, and disease activity in a longitudinal cohort (30 patients with SLE) over a 10-year period.
- To evaluate whether the presence of neutralizing anti-IFN-Abs is associated with a normalization of the B cell frequencies and their responses, we quantified ex vivo B cell subset frequencies in patient groups defined by the presence or absence of neutralizing and non-neutralizing anti-IFN-Abs and controls.
RESULTS
- Due to the well-established role of IFNα in promoting SLE pathogenesis, we focused our attention on the cohort of patients that displayed anti-IFN-Abs. Of note, anti-IFN-Abs were predominantly of the immunoglobulin G1 (IgG1) subclass. Serum samples with high anti-IFN-Ab levels were more efficient in blocking all tested subtypes (IFNα2, -5, -6, and -8) of IFNα bioactivity in vitro. Only anti-IFN-Abs with a neutralizing capacity of IC50 >100 negatively correlated with serum levels of IFNα.
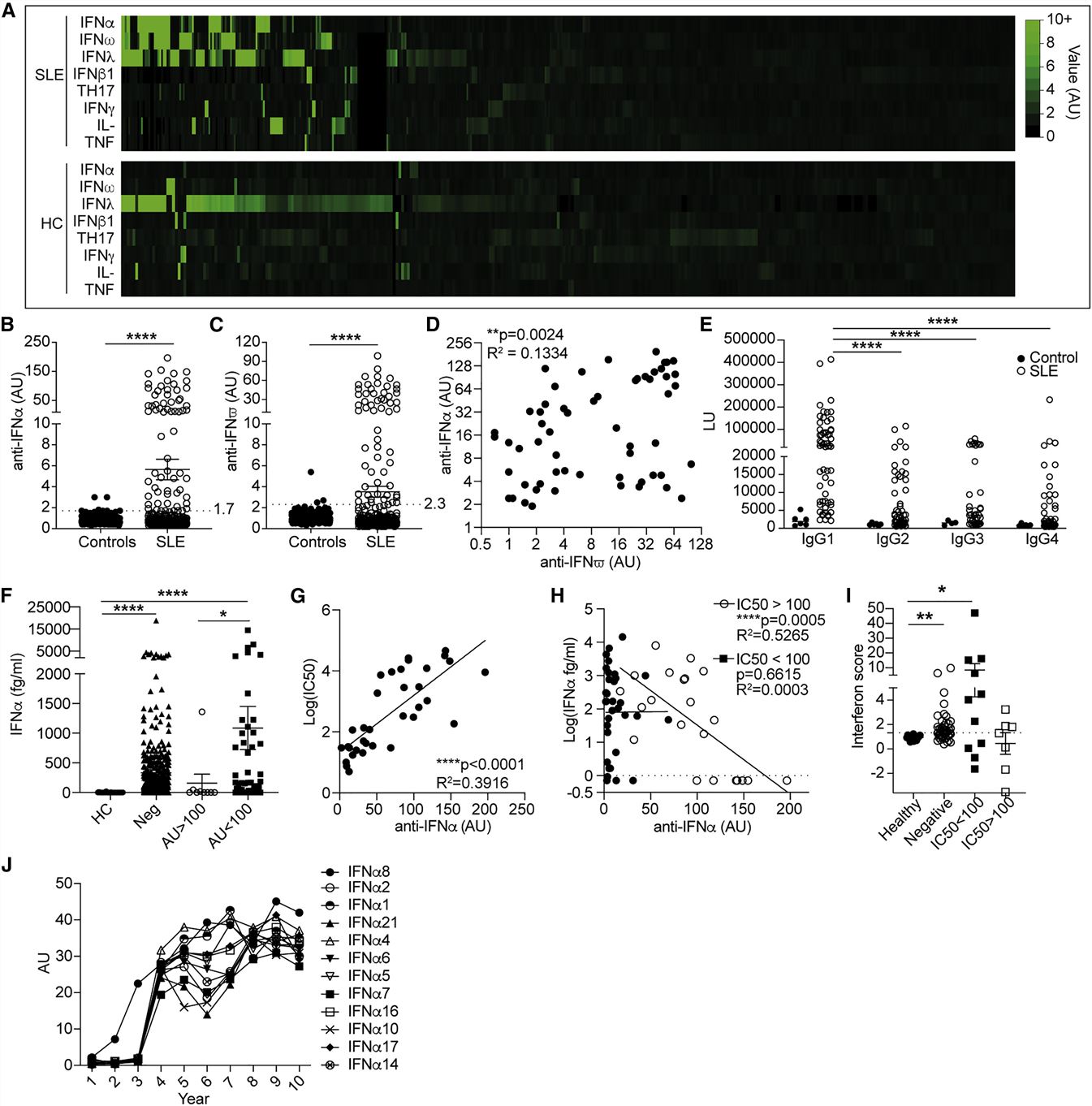 Fig. 1 Neutralizing anti-IFN-Abs in patients with SLE inversely correlate with circulating IFNα.
Fig. 1 Neutralizing anti-IFN-Abs in patients with SLE inversely correlate with circulating IFNα.
- We next investigated the effect that the presence of neutralizing anti-IFN-Abs has on disease severity. Patients with at least one neutralizing Ab against an IFNα subtype displayed significantly lower disease activity compared with patients without anti-IFN-Abs in circulation or patients with non-neutralizing anti-IFN-Abs. The prolonged presence of neutralizing anti-IFN-Abs together with a consistently low IFNα concentration also paralleled a persistent inactive clinical score. Patients with non-neutralizing anti-IFN-Abs in circulation present with high levels of serum IFNα and a more severe disease activity. Patients lacking anti-IFN-Abs present active disease over time.
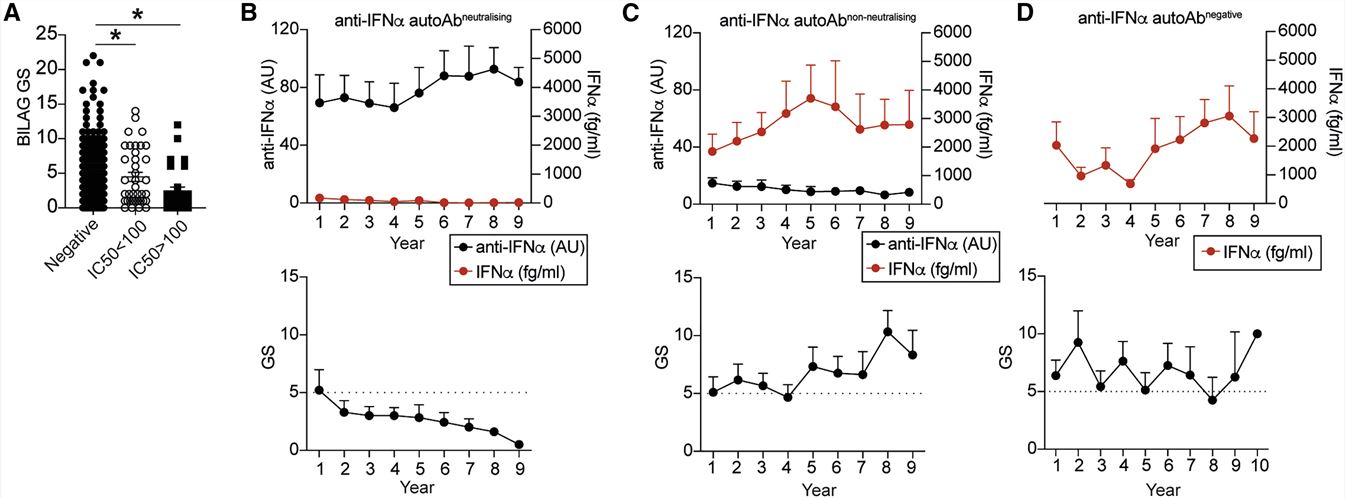 Fig. 2 Neutralizing anti-IFN-Abs are longitudinally stable, neutralize IFNα in vivo, and are associated with inactive disease.
Fig. 2 Neutralizing anti-IFN-Abs are longitudinally stable, neutralize IFNα in vivo, and are associated with inactive disease.
- Anti-IFN-Ab-negative patients showed a significant increase in immature, DN (CD27−IgD−) and plasma blast (CD27+IgD−CD38hi) B cells and a reduced frequency of unswitched memory (USM; CD27+IgD+) and class-switched memory (CD27+IgD−CD38low) B cells compared with controls. Patients with non-neutralizing (IC50 < 100) anti-IFN-Abs display the same degree of altered subset frequencies as anti-IFN-Ab-negative patients.
- IgG from patients containing neutralizing anti-IFN-Abs significantly downregulated ISG expression in cultured CpGC-stimulated control PBMCs, confirming their ability to inhibit IFNα downstream signaling. IgG from patients with neutralizing anti-IFN-Abs reduced the levels of IFNα in culture supernatants, whereas non-neutralizing anti-IFN-Abs increased IFNα concentrations. IgG isolated from patients with neutralizing anti-IFN-Abs significantly reduced the expansion of immature B cells and plasma blasts, with the latter also confirmed by a reduced Blimp1 expression, compared with non-neutralizing anti-IFN-Abs.
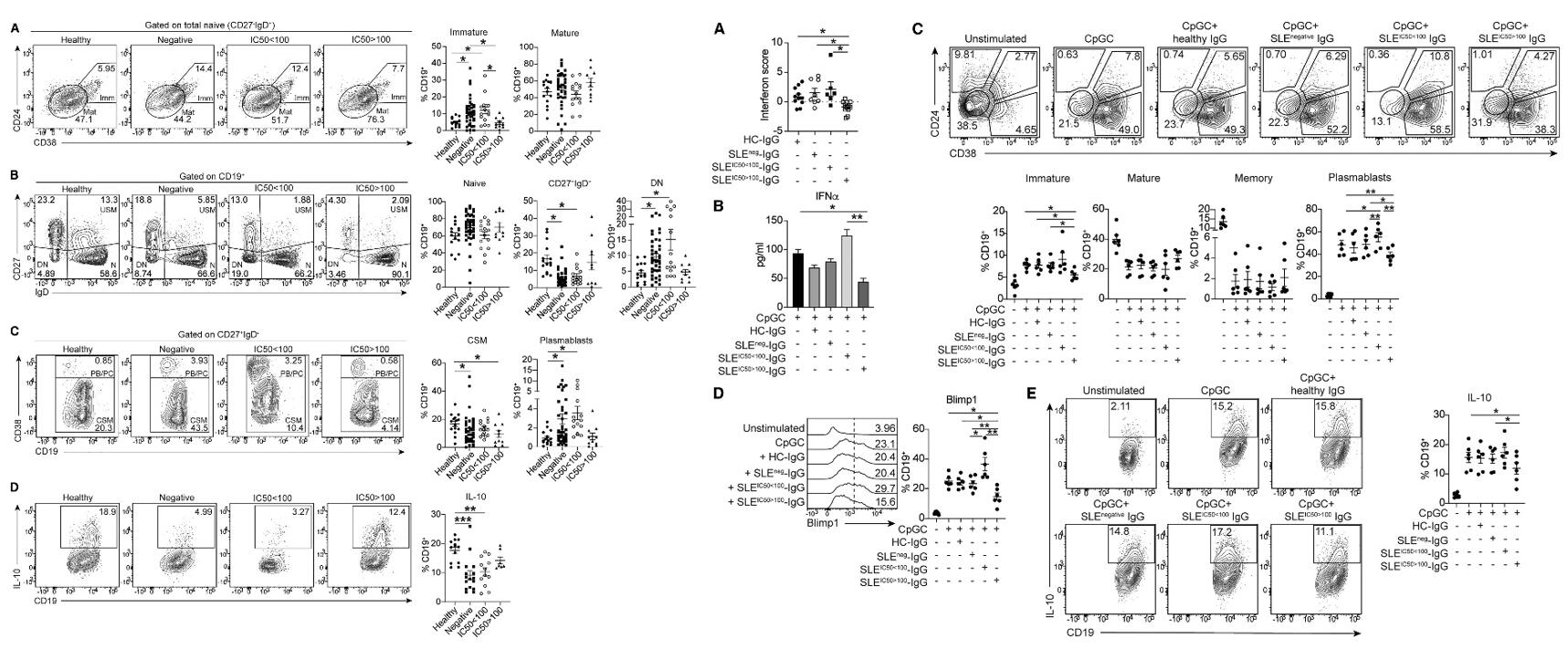 Fig. 3 Left: Patients with SLE with neutralizing anti-IFN-Abs display normalized frequencies of peripheral blood B cell subsets; Right: IgG from patients with neutralizing anti-IFN-Abs inhibits healthy B cell responses.
Fig. 3 Left: Patients with SLE with neutralizing anti-IFN-Abs display normalized frequencies of peripheral blood B cell subsets; Right: IgG from patients with neutralizing anti-IFN-Abs inhibits healthy B cell responses.
SUMMARY
- Immunoglobulin G (IgG) purified from sera of patients with SLE with neutralizing anti-IFN-Abs impedes CpGC-driven IFNα-dependent differentiation of B cells into immature B cells and plasma blasts, thus recapitulating the neutralizing effect of anti-IFN-Abs on B cell differentiation in vitro.
- Our findings highlight a role for neutralizing anti-IFN-Abs in controlling SLE pathogenesis and support the use of IFN-targeting therapies in patients with SLE lacking neutralizing-anti-IFN-Abs.
RELATED PRODUCTS & SERVICES
Reference
- Bradford HF, et al. (2023). "Inactive disease in patients with lupus is linked to autoantibodies to type I interferons that normalize blood IFNα and B cell subsets." Cell Rep Med. 4 (1), 100894.