Exosome Secretion and Uptake Involved in Cancer Therapy
Cancers (Basel). 2023 Apr; 15 (7): 1992.
Authors: Lau NCH, Yam JWP.
INTRODUCTION
Exosomes are nanometer-sized vesicles released by different cells that are important in the normal functioning of the body. In cancer, exosomes have been found to promote tumor growth and metastasis by carrying functional biomolecules and acting on different target sites in the body. Understanding the mechanism by which cancers modulate exosome secretion is crucial to studying cancer biology and developing new therapeutic approaches.
Regulation of Exosome Secretion in Cancer
- Exosomes promote cancer development by priming premetastatic niches, evading immune surveillance, and developing resistance to therapy. To this effect, various mechanisms are utilized by cancer cells to regulate the amount and composition of exosomes.
- Exosome biogenesis is directly affected by the aberrant expression of exosome biogenesis machinery. For the formation of MVBs, the proteins Hrs, Hsp90, and Syntenin act as oncogenes to promote exosome release and promote cell invasion in cancer cells. Dysregulation of molecular signaling pathways in cancer has also been found to modulate exosome release, such as the Ras/Raf/MEK/ERK, Ca2+, glutamine, p53, mTOR, STAT3, and GPCR signaling pathways.
The Role of Exosome Targeting in Cancer and Drug Delivery
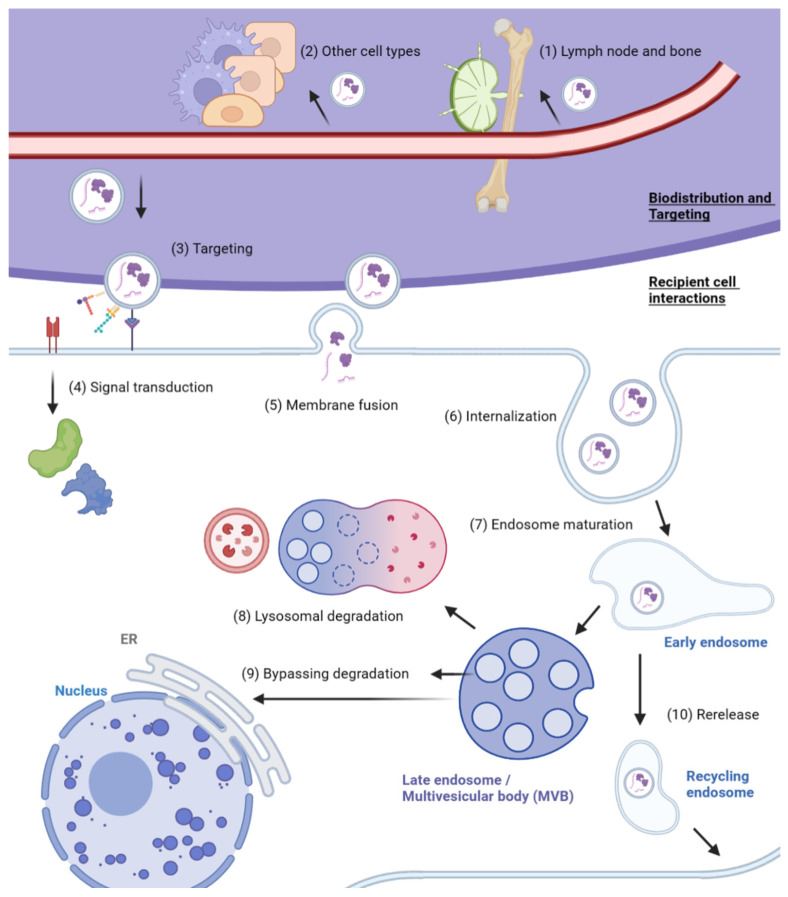 Fig. 1 Overview of exosome biodistribution and uptake. (Lau NCH and Yam JWP, 2023)
Fig. 1 Overview of exosome biodistribution and uptake. (Lau NCH and Yam JWP, 2023)
- In addition to the variety of tumorigenic and metabolic effects induced in tumor cells, exosomes also mediate the crosstalk of tumor cells, the extracellular matrix, stromal cells, and immune cells. Exosomes regulate the macrophage polarization between M1 and M2 phenotypes and can induce the transformation towards M2 macrophages that promote tumor growth. Tumor-derived exosomes also act on T cells, B cells, and natural killer (NK) cells, activating immune responses while contributing to immune evasion.
- The study of exosome targeting and uptake is especially relevant to their use as drug-delivery vehicles. To generate exosomes that display desired surface molecules, genetic engineering or chemical engineering approaches have been employed. Genetic engineering refers to the incorporation of ligand-expressing plasmids into donor cells, while chemical engineering involves chemical conjugation or electroporation of collected exosomes. Genetic engineering is more favorable towards exosome integrity, as it utilizes the natural biogenesis pathways, whereas direct modification methods may negatively impact the stability of the membrane structure, as well as the endogenous and exogenous targeting molecules, which hinders the targeting effectiveness of the produced exosomes.
Cancer Exosomes as a Therapeutic Target
- Cancer-specific dysregulation of exosome biogenesis has given rise to the pharmacological inhibition of exosome machinery as a potential avenue of treatment. To inhibit exosome biogenesis and release, pathways, such as nSMase2, Syntenin, and Ras signaling, have been targeted. Besides exosome biogenesis, exosome transport can also be inhibited via various approaches. Finally, EV uptake inhibitors have been explored to reduce cancer-derived EV uptake by both cancer and non-cancer cells, with the aim of reducing EV-mediated chemoresistance, proliferation, and metastasis.
- To select drugs for clinical translation, the solubility, and potency of inhibitors should be adequately tested. Currently, there are difficulties in clinical translation in terms of drug efficacy, drug specificity, and gaps in mechanistic knowledge. While some drugs target only one protein in EV biogenesis, other drugs may have multiple targets. While inhibitors that target multiple pathways may be more efficacious, their downstream effects are more difficult to control precisely.
RELATED PRODUCTS & SERVICES
Reference
- Lau NCH and Yam JWP. (2023). "From Exosome Biogenesis to Absorption: Key Takeaways for Cancer Research." Cancers (Basel). 15 (7), 1992.