Engineering Stem Cells in Cancer Immunotherapy
Cell Stem Cell. 2023 Mar 13; S1934-5909(23), 00070-X.
Authors: Li YR, Dunn ZS, Yu Y, Li M, Wang P, Yang L.
INTRODUCTION
Two unique properties of stem cells, namely self-renewal and the ability to differentiate into multiple cell types, make them an attractive source for cell-based therapies. Stem cells and stem cell-derived products have been investigated for the treatment of a variety of diseases and also provide an attractive paradigm for cancer immunotherapy.
Stem cells are designed to stably express various chimeric antigen receptors (CARs) or T-cell receptors (TCRs) targeting tumor-associated antigens, showing increasing promise in the treatment of solid tumors and hematologic malignancies. Stem cell transplantation generates long-term immune cells and serves as a continuous source of tumor-specific effector cells to maintain remission. In addition, stem cell engineering provides "off-the-shelf" cellular products, avoiding the need for personalized and patient-specific products that plague current autologous cell therapies.
iPSC-Derived NK Cells
- The use of iPSC-derived NK cells is receiving increased interest. iPSCs are a renewable cell source that can be expanded indefinitely to produce homogeneous NK cells, addressing the manufacturing and supply chain bottlenecks associated with primary NK cells. Preclinically, iPSC-NK cells have shown powerful antitumor functions against a variety of cancers in xenograft models.
- In a representative study, non-KIR expressing NK cells derived from donor peripheral blood-iPSCs had greater cytotoxicity against ovarian cancer, colorectal cancer, breast cancer, and head and neck cancer cells compared with primary NK cells.
iPSC-Derived Immune Cells
In 2013, two Japanese research groups generated rejuvenated iPSC-derived, antigen-specific T cells. Using a similar technology, human MAIT cells are reprogrammed into iPSCs and re-differentiated the iPSCs to MAIT cells with antimycobacterial activity. In the same year, combining T-iPSC and CAR technologies to develop CD19 CAR-T to treat B cell malignancies. Following the T-iPSC technology, several groups generated rejuvenated iNKT, NK cells, and DCs111 from reprogrammed iPSCs.
iPSC-Derived CAR-T Cells
iPSC-Derived CAR-T cells have the potential to be an infinite source of phenotypically defined, expandable, and functional CAR-T cells for off-the-shelf cancer therapy. However, compared with iPSC-NK cells, the generation of iPSC-derived CAR-T cells have been challenging and typically requires a preexisting TCR that directs in vitro T cell differentiation. In addition, T cell differentiation requires notch ligand engagement and can be impaired by CAR expression.
iPSC-Derived iNKT Cells
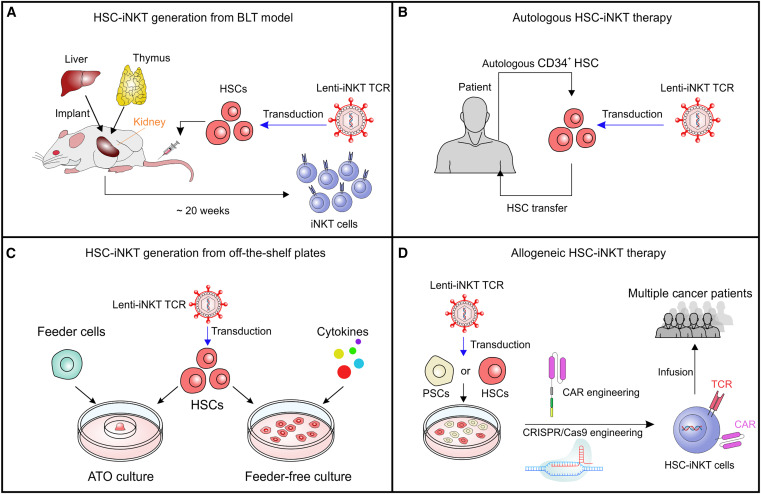 Fig. 1 Development of HSC-engineered iNKT (HSC-iNKT) cell therapies for cancer HSC-iNKT cells. (Li YR, et al., 2023)
Fig. 1 Development of HSC-engineered iNKT (HSC-iNKT) cell therapies for cancer HSC-iNKT cells. (Li YR, et al., 2023)
- iNKT cells are another potentially promising cell population for cancer immunotherapy. However, the low frequency and high variability of iNKT cells in humans limit their clinical applications. To overcome these challenges, an HSC-iNKT cell platform was developed.
- Subsequent development of the HSC-iNKT cell platform has centered on producing off-the-shelf HSC-iNKT cells, and it was recently shown that HSC engineering followed by in vitro differentiation resulted in allogeneic HSC-iNKT cells with high yield and purity. Allogeneic HSC-iNKT cells have innate cancer-killing capacity and a low risk of GvHD, and to enhance their therapeutic potential.
RELATED PRODUCTS & SERVICES
Reference
- Li YR, et al. (2023). "Advancing cell-based cancer immunotherapy through stem cell engineering." Cell Stem Cell. S1934-5909 (23), 00070-X.