Characterization of Transplantation of Human iPSC Neuronal Precursors
Cell Transplantation. 2023 Jan-Dec; 32: 9636897221107009.
Authors: Kobayashi Y, Shigyo M, Platoshyn O, Marsala S, Kato T Jr, Takamura N, Yoshida K, Kishino A, Bravo-Hernandez M, Juhas S, Juhasova J, Studenovska H, Proks V, Driscoll SP, Glenn TD, Pfaff SL, Ciacci JD, Marsala M.
INTRODUCTION
Previous preclinical and clinical studies using fetal tissue-derived or embryonic stem cell-derived neural precursor cells (NPCs) have established the rationale for the use of cell-replacement therapies for the treatment of a variety of spinal neurodegenerative disorders. Consistent long-term engraftment and differentiation to all neural cell types (neurons, astrocytes, and oligodendrocytes) were seen after grafting multilineage NPCs.
METHODS
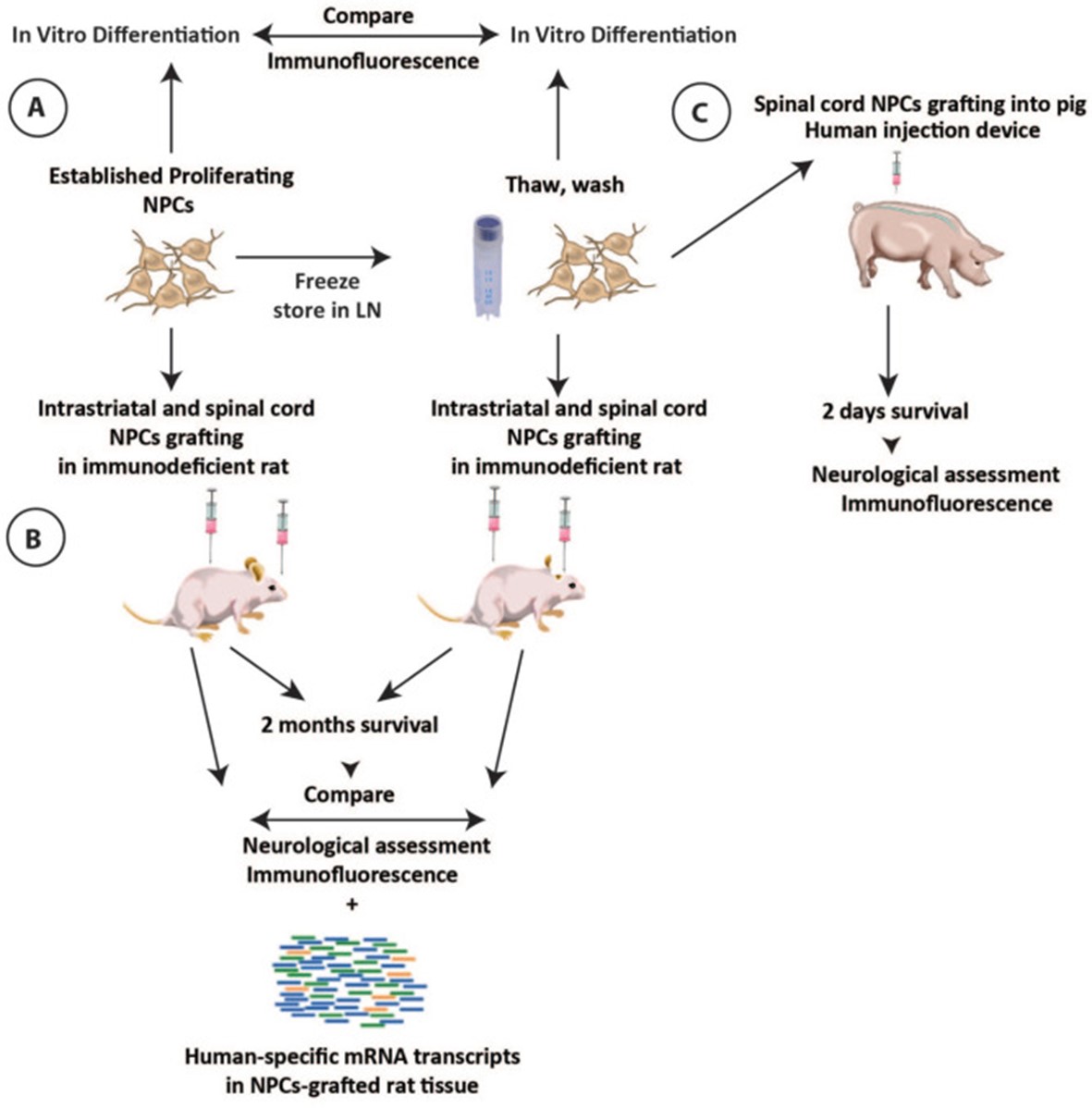 Fig. 1 Schematic diagram of the experimental design.
Fig. 1 Schematic diagram of the experimental design.
- Preparation of NPCs for in vivo grafting. Previously frozen NPCs were thawed quickly in the water bath (37°C) and transferred to 15cc of hibernation media for washing. The cell pellet was then re-suspended with a hibernation medium and filtered through 40 μm plastic mesh and a targeted cell density solution was prepared.
- In vitro NPCs differentiation and indirect immunofluorescence. Proliferating or previously frozen-washed NPCs were plated onto glass chamber slides and induced to terminally differentiate by the withdrawal of bFGF and adding 10 ng/ml BDNF and 10 ng/ml GDNF into culture media for 2 weeks. After differentiation, cells were fixed with 4% paraformaldehyde, stained with neuronal and glial markers, and images were captured and analyzed.
- In vivo cell grafting. Animals received 10 to 15 spinal NPC injections (0.5 µl each) distributed bilaterally between L2 and L6 spinal segments (20,000-30,000 viable cells per injection; depth of injections from dorsal spinal cord surface: 1 mm) using a homemade glass capillary. Each animal received both fresh and frozen NPCs injected either into the left or right side of the spinal cord, respectively. Each animal received an identical number of fresh and frozen NPCs injections.
- In qualitative and quantitative analysis of combined antibodies-stained sections, six sections (three brain and three spinal cord sections) taken from each animal with identified grafts were used. Sections were stained with hNUMA antibody in combination with neuronal and glial markers including DCX, NeuN, hGFAP, vimentin, Olig2, and Ki67. The total number of double-stained grafted cells was then counted and expressed as % of the total hNUMA-stained cell population.
- Browse our recommendations
Creative Bioarray provides professional products and services, including but not limited to the following.
| Product/Service Types | Description |
| iPSC Reprogramming Kits | Creative Bioarray offers a broad range of kits and related reagents that are useful to stem cell scientists interested in performing reprogramming trials. |
| iPSC Characterization | Creative Bioarray provides comprehensive iPSC characterization services for customers all over the world. |
| Immunohistochemistry (IHC), Immunofluorescence (IF) Service | By providing high-quality immunohistochemistry (IHC) and immunofluorescence (IF) services to customers for many years, Creative Bioarray will give you comprehensive service in regular and customized IHC and IF services. |
| Special Staining Services | Creative Bioarray can offer a variety of comprehensive and reliable special staining for sectioned paraffin-embedded tissues or cells to fit all individual needs. |
RESULTS
- Using brightfield microscopy, a similar morphological appearance of continuously cultured NPCs versus freshly plated NPCs from frozen stock (2 days post-plating) was observed before the differentiation. Proliferating and induced or previously frozen and induced NPCs were analyzed with neuron-specific and glia-specific markers at 2 weeks after in vitro induction using differentiation media.
- An array of known cellular markers was used to profile the human-derived transcripts. Consistent with the immunofluorescent analysis of spinal cord and striatal grafts, we detected the clear presence of human-specific transcripts corresponding to neuronal, astrocyte, and oligodendrocyte lineage markers. We next probed for markers of specific neurotransmitters and neuronal subtype identity, which indicated that grafted NPCs had differentiated into multiple neuronal subtypes, including both inhibitory and excitatory neurons.
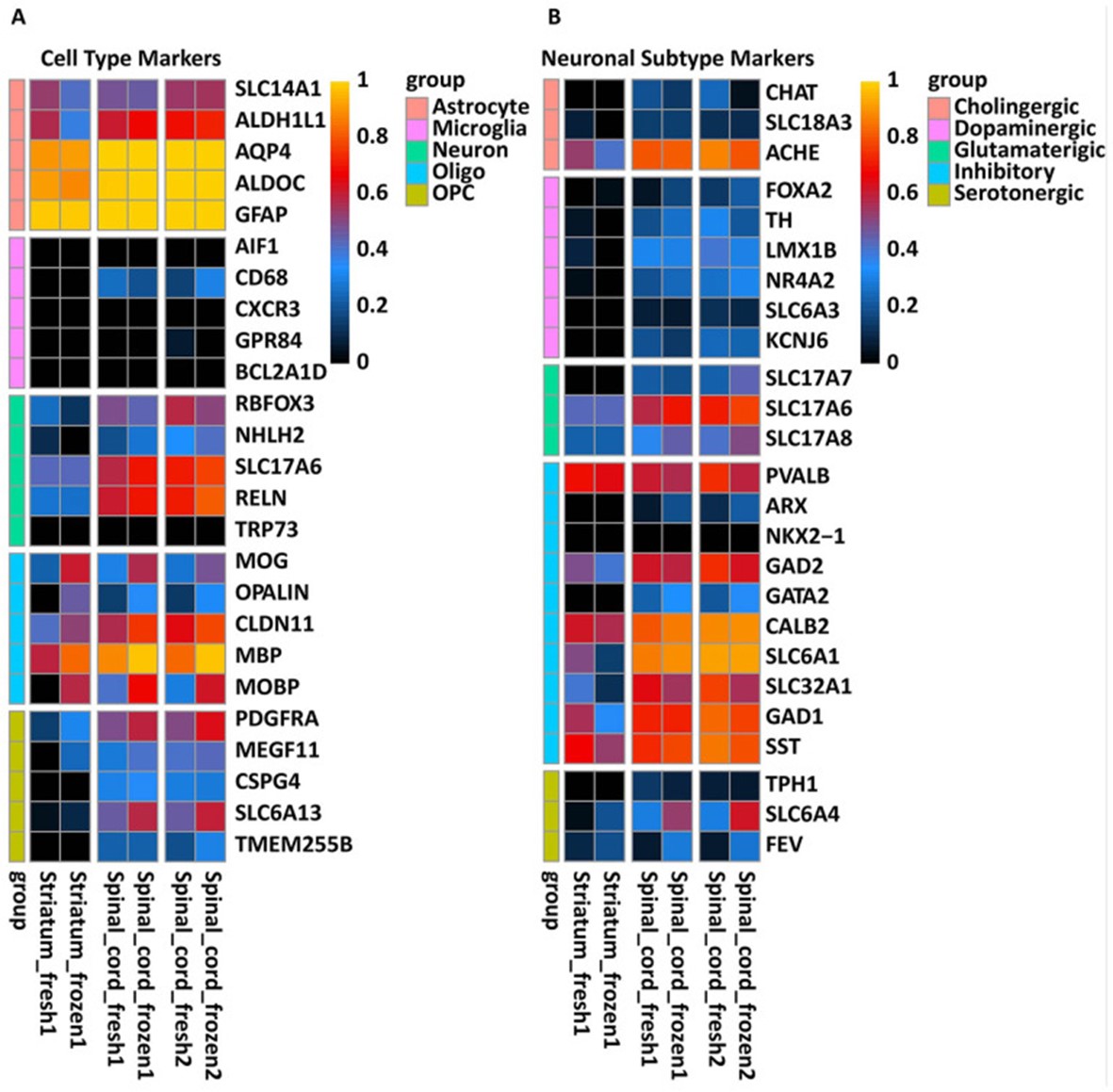 Fig. 2 Expression of human-specific neuronal and glial lineage transcripts in rat striatal and spinal cord 6 months after NPCs grafting.
Fig. 2 Expression of human-specific neuronal and glial lineage transcripts in rat striatal and spinal cord 6 months after NPCs grafting.
SUMMARY
Here, we compared the engraftment properties of established human-induced pluripotent stem cells (hiPSCs)-derived neural precursor cell (NPCs) line once cells were harvested fresh from the cell culture or previously frozen and then grafted into striata or spinal cord of the immunodeficient rat. A newly developed human spinal injection device equipped with a spinal cord pulsation-cancelation magnetic needle was also tested for its safety in an adult immunosuppressed pig.
RELATED PRODUCTS & SERVICES
Reference
- Kobayashi Y, et al. (2023). "Expandable Sendai-Virus-Reprogrammed Human iPSC-Neuronal Precursors: In VivoPost-Grafting Safety Characterization in Rats and Adult Pig." Cell Transplant. 32, 9636897221107009.