A Novel in Vitro Model of Intestinal Epithelial Drug Permeability
Pharmaceutics. 2023 Sep 18; 15 (9): 2338.
Authors: Cheng Y, Watanabe C, Ando Y, Kitaoka S, Egawa Y, Takashima T, Matsumoto A, Murakami M.
INTRODUCTION
The intestinal epithelial Caco-2 cell monolayer is a well-established in vitro model useful for predicting intestinal drug absorption in humans. Coculture models of Caco-2 and goblet-cell-like HT29-MTX cells have been developed to overcome the lack of a mucus layer; however, those models are much leakier compared to the intestinal epithelium. Here, we developed a partially laminated culture model where HT29-MTX cells were superimposed onto a Caco-2 monolayer to overcome this issue.
METHODS
- Caco-2 cells were cultured in DMEM supplemented with 10% FBS, 1% nonessential amino acids, and 1% penicillin-streptomycin. HT29-MTX cells were cultured in DMEM containing 10% FBS and 1% nonessential amino acids. Both cell lines were cultured under 5% CO2, and 95% relative humidity at 37°C using a cell culture incubator, and the media were changed every 2-3 days. Caco-2 and HT29-MTX cells were used from passages 20 to 65 and 19 to 60, respectively.
- When preparing the partially laminated model, a Caco-2 monolayer with a TEER value ≥ 2000 Ω·cm2 was formed first, and HT29-MTX cells were then laminated onto the Caco-2 monolayer at ratios of 1:9, 3:7, and 5:5, followed by incubation for the predetermined time. As for the coculture model, Caco-2 and HT29-MTX cells were seeded simultaneously in different ratios and incubated for the predetermined time to form a hybrid monolayer.
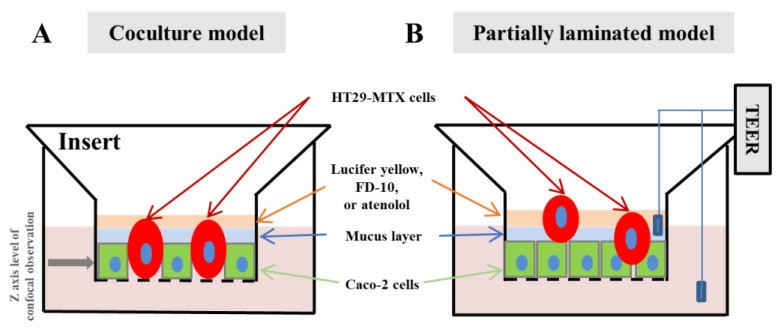 Fig. 1 Schematic images of the partially laminated model.
Fig. 1 Schematic images of the partially laminated model.
- The cell sheet obtained in each culture model was histologically examined via live-cell, hematoxylin, eosin (H&E), and PAS staining. Briefly, for live cell staining, live cells were incubated with cell trackers in serum-free medium for 45 min, followed by 30 min of incubation in regular medium.
- In addition to PAS staining, mucin formation in the cell sheet obtained in each culture model was estimated through MUC2 staining. The integrity of the cell sheet in each culture model was examined with culture time using TEER obtained with the epithelial volt-ohmmeter. The permeability of each culture membrane was determined using Lucifer yellow (MW, 521.57), FITC-dextran (average MW, 10 kDa), and atenolol (MW, 266.34) as a model drug.
- Browse our recommendations
| Product/Service Types | Description |
| Classic media | Creative Bioarray, with decades of expertise, provides high-quality classical cell culture media to support research and biotechnology endeavors. |
| Histological Stains & Dyes | Creative Bioarray provides a series of histological staining methods and related products to help our clients achieve the best dyeing purposes. |
| Histology Services | Creative Bioarray provides our global clients with the most comprehensive histology services. We offer tissue processing, embedding, sectioning, and staining. |
| In Vitro Permeability and Transporters | Creative Bioarray provides several in vitro permeability and transporter assays to evaluate drug permeability and predict drug absorption and distribution. |
| Caco-2 Permeability Assay | Creative Bioarray is offering Caco-2 permeability assays to help determine the absorption and the bioavailability of drug candidates, facilitating the lead optimization process in drug discovery. |
RESULTS
- Caco-2 cells formed a compact and thin monolayer, which represented the intestinal epithelial cell layer. In contrast, HT29-MTX cells appeared to form a much looser and thicker layer, with the mucin granules clustered in the cells, showing the morphological characteristics of mature goblet cells. Second, we evaluated the morphological differences between the partially laminated and coculture models. The most significant differences in the Caco-2 layer between the two models were seen at an HT29-MTX ratio of 50%.
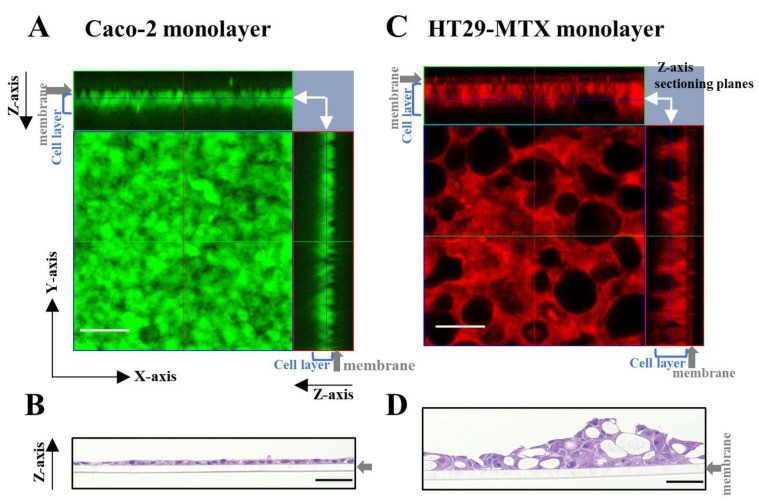
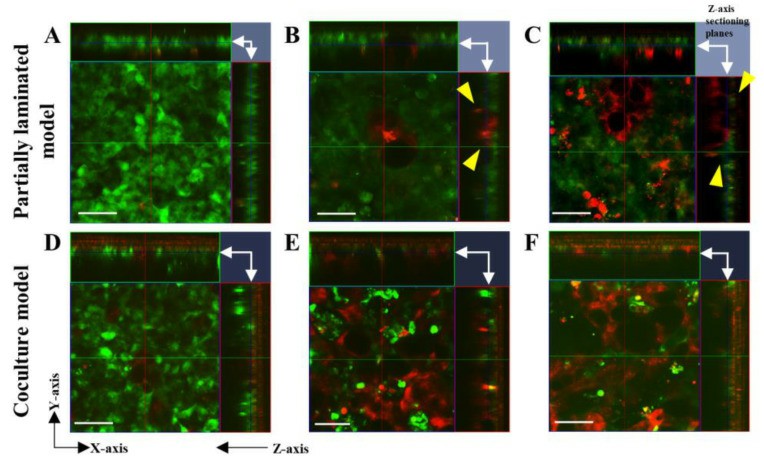
Fig. 2 Left: Morphology assessment of Caco-2 and HT29-MTX monocultures using live cell and hematoxylin and eosin (H&E) staining. Right: Morphology evaluation of the Caco-2/HT29-MTX partially laminated and coculture models using live cell staining.
- PAS and H&E staining in the continuous section showed that the layers of the partially laminated model were thicker than the Caco-2 monolayer, and some areas of the cytoplasm and surface were positively stained in a strong purple color. MUC2 was found at the apical surface and mid-depth in the HT29-MTX monolayer, but not as much in the Caco-2 monolayer and basal depth in the HT29-MTX monolayer. MUC2 expression increased proportionally to the ratio of HT29-MTX in the partially laminated model.
- TEER values were extremely lower for the coculture model at 7:3 and 5:5 ratios of Caco-2/HT29-MTX cells. Even at a 9:1 ratio, the TEER values were dramatically lower than those in the Caco-2 monolayer. These results indicate that the TEER of the partially laminated model was lower than that of the Caco-2 monolayer, but they could maintain a tight barrier function in the epithelial layer. In the coculture model, TEER was dramatically lower even at a 7:3 ratio of Caco-2/HT29-MTX cells.
SUMMARY
A UHPLC-UV method was successfully validated for the detection and quantification of MDPV in the transport buffer of this study, HBSS (+/+). The results showed that the enantiomers of MDPV were highly permeable across the Caco-2 monolayer. Enantioselectivity was found between the enantiomers in the Papp values obtained for the BL to AP direction, suggesting the involvement of a transport protein. Efflux ratios indicate that a facilitated diffusion mechanism should be accepted for the efflux of the enantiomers of MDPV.
RELATED PRODUCTS & SERVICES
Reference
- Cheng Y, et al. (2023). "Caco-2 Cell Sheet Partially Laminated with HT29-MTX Cells as a Novel In Vitro Model of Gut Epithelium Drug Permeability." Pharmaceutics. 15 (9): 2338.