Reprogramming of Human Fibroblasts into iPSCs
Reprogramming of somatic cells into pluripotent stem cells has been reported by introducing a combination of several transcription factors (Oct3/4, Sox2, Klf4 and c-Myc). The induced pluripotent stem (iPS) cells from patient's somatic cells could be a useful source for drug discovery and cell transplantation therapies. To date, most iPS cells were made by using viral vectors, such as retroviruses and lentiviruses. Here we describe a stepwise protocol for the generation of modified virus-derived iPS cells from primary human fibroblasts.
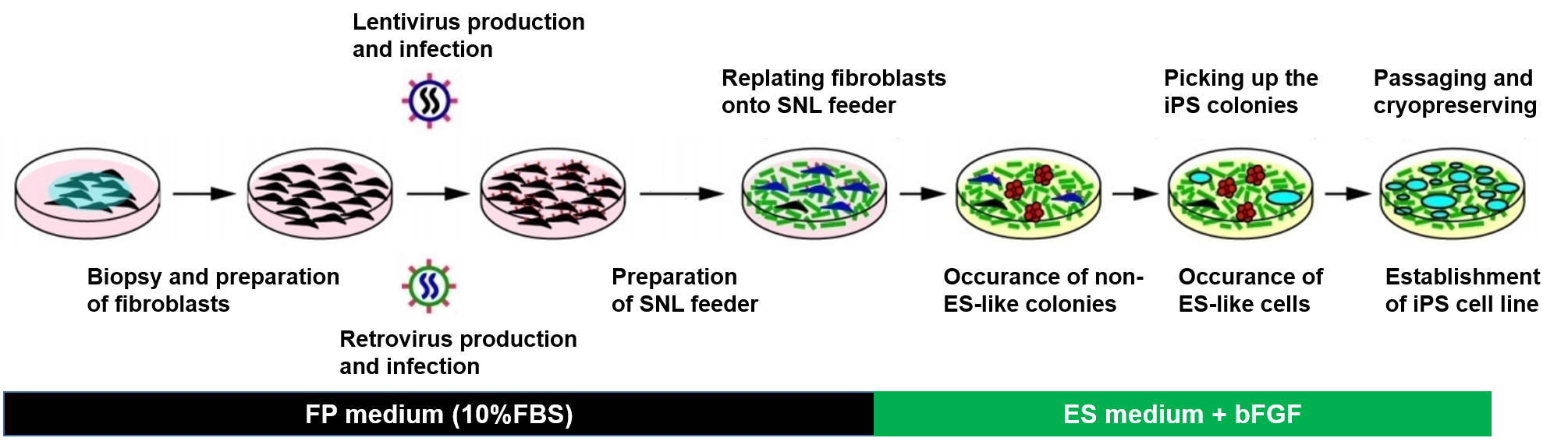 Figure 1. Schematic diagram of human iPS cell generation.
Figure 1. Schematic diagram of human iPS cell generation.
Materials and Reagents
| Lentivirus packaging mix | Lentiviral expression system |
| pMXs retroviral vectors and Plat-E packaging cells | pLenti6/UbC containing mouse Slc7a1 gene |
| SNL feeder cells | 293FT cells |
| 0.25% Trypsin/1 mM EDTA solution | 0.5% Trypsin/5.3 mM EDTA solution |
| Collagenase IV | Gelatin |
| Human ES medium (DMEM/F12 containing 20% KSR, 2 mM L-glutamine, 1x 10-4 M non-essential amino acids, 1x 10-4 M 2-mercaptoethanol) | 293FT medium (DMEM containing 10% FBS, 2 mM L-glutamine, 1x 10-4 M non-essential amino acids, 1 mM sodium pyruvate) |
| SNL medium (DMEM containing 7% FBS, 2 mM L-glutamine) | FP medium (for fibroblasts and Plat-E cells) |
| OPTI-MEM I | Lipofectamine 2000 |
| Phosphate-buffered saline (without Ca2+, Mg2+) | Fugene 6 transfection reagent |
Generation of iPS Cells with Lentivirus
- Lentivirus production
1) Dilute 9 μg of packaging mix (pLP1, pLP2 and pLP/VSVG mixture) and 3 µg of pLenti6/UbC encoding the mouse Slc7a1 gene in 1.5 mL of OPTI-MEM I, and mix gently.
2) In a separate tube, dilute 36 µL of Lipofectamine 2000 in 1.5 mL of OPTI-MEM I. Mix gently and incubate for 5 min at room temperature.
3) After incubation, combine the diluted DNA with the diluted Lipofectamine 2000. Mix gently by finger tapping and incubate for 20 min at room temperature.
4) During incubation, remove the medium from 293FT dishes, and add 9 mL of fresh medium to each dish.
5) Then add 3 mL of the DNA-Lipofectamine 2000 complexes in each dish. Mix gently by rocking the dish back and forth. Incubate the dish at 37˚C, 5% CO2.
6) 48 hours after transfection, collect the supernatant of the 293FT culture, and then filtrate it with a 0.45 µm pore size cellulose acetate filter.
Note: The lentivirus-containing medium can be stored at -80˚C. Do not repeat freeze/thaw cycles to avoid the reduction of viral titer.
- Lentiviral infection
1) Prepare primary human fibroblasts from skin biopsy specimens or purchase cells from vendors.
2) Replace medium with 10 mL/dish of the virus-containing supernatant, supplemented with 4 μg/mL polybrene. Incubate the dishes for 5 hours to overnight at 37˚C, 5% CO2. 24 hours after transduction, aspirate off the virus-containing medium, wash cells once with 10 mL of PBS (optional), and add 10 mL of fresh FP medium.
Note: a) 3 to 5 days after infection, transgene expression of lentivirus should reach the maximum level. b) You may use the cells immediately or freeze them at -80˚C for storage.
Generation of iPS Cells with Retrovirus
- Plat-E preparation
1) Wash the cells with PBS, add 4 mL of 0.05% trypsin/0.53 mM EDTA.
2) After incubation, add 10 mL FP medium (contains neither puromycin nor blasticidin S), suspend the cells by gently pippeting, and transfer the cell suspension to a 50 mL conical tube.
3) Centrifuge the cells at 180 g for 5 minutes, and then discard the supernatant, resuspend the cells in an appropriate amount of FP medium.
4) Count cell number and adjust the concentration to 3.6 x 105 cells/mL with FP medium.
5) Seed cells into 100 mm dish, and incubate overnight at 37˚C, 5% CO2.
- Retrovirus production
1) Transfer 0.3 mL of OPTI-MEM I into a 1.5 mL tube.
2) Deliver 27 μL of Fugene 6 transfection reagent, mix gently and incubate for 5 minutes at room temperature.
3) Add 9 μg of pMXs plasmid DNA (respectively encoding Oct3/4, Sox2, Klf4, and c-Myc) drop-by-drop into the Fugene 6/OPTI-MEM I containing tube, mix gently and incubate for 15 minutes.
Note: Introduce one plasmid into one dish. Transfection of more than two plasmids into a dish causes reduction of efficiency of iPS cell generation.
4) Add the DNA/Fugene 6/OPTI-MEM I complex drop-wise into the Plat-E dish, and incubate at 37˚C, 5% CO2.
Note: Also transfect with a suitable control; we use pMXs retroviral vector GFP to monitor transfection efficiency. High efficient transfection is crucial for iPS cell induction.
- Retroviral infection
1) 48 hours post-transfection, collect the medium from each Plat-E dish, filtering it through a 0.45 μm pore size cellulose acetate filter, and transferring into a 15 mL conical tube.
2) Add 5 μL of 8 mg/mL polybrene solution into the filtrated virus-containing medium, and mix gently. The final concentration of polybrene is 4 μg/mL.
3) Make a mixture of equal parts of the medium containing Oct3/4-, Sox2-, Klf4- and c-Myc-retroviruses.
Note: Retroviruses should be used freshly! Do not freeze, or you won’t obtain iPS cells. The titer of retrovirus is critical for iPS cell generation. The freeze/thaw step decreases the titer of retrovirus.
4) Aspirate the medium from fibroblast dishes, and add 10 mL per dish of the polybrene/virus-containing medium. Incubate the cells at 37˚C, 5% CO2.
5) 48 hours after infection, aspirate off the virus-containing medium, and add 10 mL of fresh FP medium.
Mitomycin C-inactivation of SNL Cells
1) Add 0.3 mL of 0.4 mg/mL mitomycin C solution directly to the culture medium of SNL dish, swirl briefly and incubate 2 hours at 37˚C, 5% CO2. The final concentration of mitomycin C will be 12 μg/mL.
2) After incubation, aspirate the mitomycin C-containing medium off the cells, and wash the cells twice with 10 mL of PBS.
3) Add 0.5 mL of 0.25% trypsin/1 mM EDTA, incubate for 1 minute at room temperature.
4) Neutralize the trypsin by adding 5 mL of SNL medium, and count the cell number.
5) Seed the cells at 1.5 x 106 cells per 100 mm dish, or at 2.5 x 105 cells per well of 6-well plate.
6) Cells should be well spread with space between each other. They should become ready for usage of the next day.
Note: The mitomycin C-treated SNL dishes can be left for up to three days before use. In addition, you can make frozen stocks of mitomycin C-treated SNL cells with a standard technique at -80˚C or in liquid nitrogen tank. These stocks should be woken up in a gelatin-coated dish or plate within the 3 days before use. Old SNL feeder (over three days after mitomycin C-treatment) may come off from the dish during over three weeks of iPS cell generation.
Replating Transduced Fibroblasts onto Mitomycin C-treated SNL Feeder
1) Aspirate the culture medium and wash with 10 mL per dish of PBS.
2) Add 1 mL of 0.05% trypsin/0.53mM EDTA, and incubate at 37˚C for 10 minutes.
3) Add 9 mL of FP medium, suspend the cells to a single cell and transfer to a 50 mL conical tube.
4) Count cell numbers, and adjust the concentration to 5 x 103 or 5 x 104 cells/mL.
5) Transfer 10 mL of cell suspension into 100 mm dish with mitomycin C-treated SNL cells. Incubate the dishes overnight at 37˚C, 5% CO2.
6) Replace the medium with 10 mL of human ES medium. Change the medium every other day until the colonies become large enough to be picked up.
Note: Colonies should become visible 2-3 weeks after the retroviral infection. They can be picked up at around day 30 when they become large enough.
Picking up the iPS Colonies
1) Remove the medium from the dishes, add 10 mL of PBS per dish.
2) Pick colonies from the dish under the stereomicroscope using a P2 or P10 Pipetman set at 2 μL, and transfer it into the 96-well plate with 20 μL of human ES medium.
3) When colony picking is finished, add 180 μL of human ES medium to each well, and pipet up and down to break up the colony to small clumps (20-30 cells) carefully under the stereomicroscope.
4) Transfer cell suspension into 24-well plates with mitomycin C-treated SNL feeder cells, add 300 μL of human ES medium per well, and incubate in a 37˚C, 5% CO2 incubator until the cells reach 80-90% confluence.
5) This usually takes 7 days. At this point they should be passaged into 6-well plates.
Troubleshooting
| Problems | |
| Fibroblasts die or do not grow well after lentiviral transduction. | In some cases, lentivirus is toxic to fibroblasts. This problem should be overcome by doubling dilution of the supernatant with the fresh medium or shorting exposure time. |
| No ES-like colonies appear after induction. | 1) Retroviruses must be prepared freshly. 2) Passage numbers of fibroblast is also critical for iPS generation. We recommend using fibroblasts within passage 8 for iPS production. 3) When seeding retrovirally transduced fibroblasts onto feeder cells, cell density is critical. Optimal cell densities are different for each fibroblast culture. Two to three different densities should be tested. |
| Isolated clone has an abnormal karyotype or is not pluripotent. | We recommend to expand multiple clones. |
| Characters and potentials of iPS cell change during culture. | Frozen stocks should be prepared at early passages (within passage number 15). |
| Cells detach from the bottoms of culture dishes during iPS cell generation. | 1) First, feeder cells may be too old. We recommend to use SNL feeder cells within 3 days after mitomycin C-treatment. 2) Another possibility is that cell density may be too high, overgrowth of fibroblasts may result in peeling off from the edge of the dish. |
| The viability of iPS cells is too low after passage or thawing. | Dissociation to single cells decreases the viability of iPS cells. Break colonies to small clumps of 20-30 cells. |
| iPS cells spontaneously differentiate. | iPS cells can be unstable during early passages, you can remove the differentiated colonies by aspirating and transfer only undifferentiated colonies to a new dish. In addition, the qualities of feeder cells, such as density and freshness, are quite important. |
References
- Mandal P.K. et al. Reprogramming human fibroblasts to pluripotency using modified mRNA. Nat Protoc, 2013, 8(3): 568-582.
- Nasir M. et al. A Review of the methods for human iPSC derivation. Methods Mol Biol, 2013, 997: 23-33.