Immunohistochemistry Protocol
(For Formalin-fixed Paraffin Embedded Tissue Samples)
Immunohistochemistry (IHC) is the process of detecting antigens (e.g. proteins) in cells of a tissue section by exploiting the principle of antibodies binding specifically to antigens in biological tissues. IHC can be used to detect protein ex
ICH-Assay Work Flow
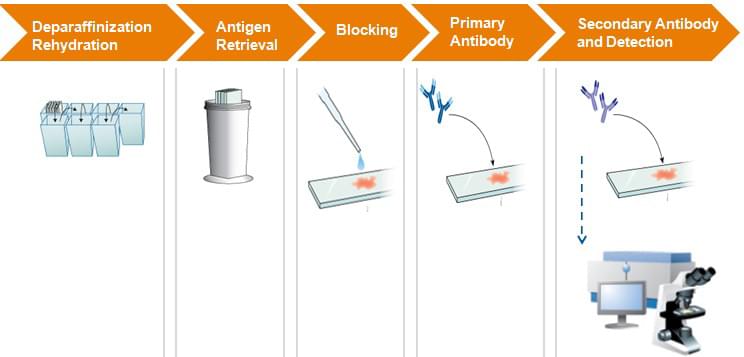
Deparaffinization Rehydration
| 1) | Sequentially immerse paraffin sections into: a. 90% dimethybenzene (for 5 min); b. 95% dimethybenzene (for 5 min); c. 100% dimethybenzene (for 5 min); d. 90% ethanol (for 5 min); e. 95% ethanol (for 5 min); f. 100% ethanol (for 5min). |
| 2) | Rinse slides twice in water for 5 min to remove ethanol. |
Antigen Retrieval (Microwave Method)
| 1) | Immerse slides in 3% H2O2 for 5 minutes. |
| 2) | Wash slides twice (2x) with distilled water (for 3-5 min). |
| 3) | Immerse slides in the working citrate buffer and cover with a lid. |
| 4) | Heat the buffer in microwave and turn it off when the buffer has boiled. |
| 5) | Remove from heat and let it stand at room temerature for 20 minutes. |
| 6) | Wash the slides 1X to 2X with PBS. |
| 7) | Remove the liquid and use a pen to circle around the tissue. |
Blocking
| 1) | Add 5% BSA blocking solution to the HIER treated samples. |
| 2) | Incubate the samples at 37°C for 30 min. |
Primary Antibody Icubation
| 1) | Dilute the primary antibody to the recommended concentration by the antibody manufactuer. |
| 2) | Add the diluted antibody to the samples and incubate overnight at 4°C or 37°C for 1h. |
| 3) | Wash slides three times (20 min) in 2X PBS each on the shaker. |
Secondary Antibody Incubation and Detection
| 1) | Dilute secondary antibody with antibody diluent to the recommended concentration by the antibody manufactuer. |
| 2) | Add the dilute antibody to the samples and incubate at 37°C for 30 min. |
| 3) | Wash slides three times (20 min each) in 2X PBS on the shaker. |
| 4) | Add Strept-Avidin Bion Complex (SABC) HRP- or AP-conjugated reagents to the samples. Incubate the samples at 37°C for 30 min. |
| 5) | Wash the slides three times (20 min each) in 3X PBS each on the shaker. |
| 6) | Add an enough amount of DAB reagent to the sampes and incubate in dark at room temperature for 10 to 30 min. |
| 7) | Wash the samples with distilled water. |
| 8) | Check the tissue staining intensity under a bright-field microscope. |