Human Nucleus Pulposus Cells (HNPC)
Cat.No.: CSC-7749W
Species: Human
Source: Cartilage
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human nucleus pulposus cells (NP cells) are primarily derived from the nucleus pulposus of intervertebral discs. In the early stages of embryonic development, the nucleus pulposus is populated by notochordal cells. As the intervertebral discs mature, the notochordal cells are replaced by smaller chondrocyte-like cells. There is also evidence that nucleus pulposus cells can be of annulus fibrosus cell origin. This may particularly be the case in the degenerative process, where cell type and morphology change.
NP cells are the major synthesizers of intervertebral disc proteoglycans, in particular aggrecan and chondroitin sulfate. The combination of proteoglycans and hyaluronic acid creates a hydrated gel-like matrix that delivers excellent shock absorption and cushioning for the nucleus. NP cells are responsive to the inflammatory factors IL-6 and TNF-α, and can themselves secrete inflammatory mediators including PGE2 and IL-8, and are thus involved in inflammatory regulation. Moreover, NP cells exhibit certain phagocytic functions, which may help in clearing cellular debris produced during the degenerative process. They also possess stem cell characteristics, enabling them to differentiate into various cell types, such as chondrocytes and fibroblasts, which enhances their potential application value in tissue engineering and regenerative medicine.
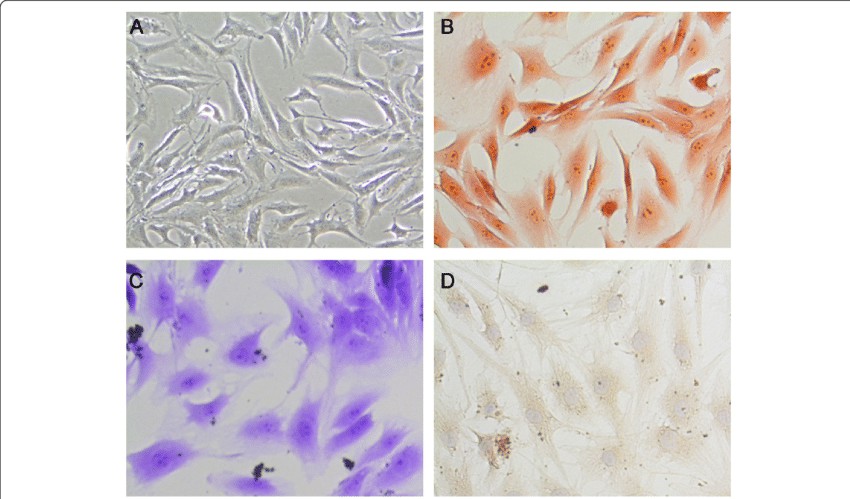 Fig. 1. A. The gross morphology of the nucleus pulposus cells. B. Safranin O staining of nucleus pulposus cells. C. Toluidine blue staining of nucleus pulposus cells. D. Immunohistochemically for collagen II of nucleus pulposus cells (Zhang C, Tong T, et al., 2021).
Fig. 1. A. The gross morphology of the nucleus pulposus cells. B. Safranin O staining of nucleus pulposus cells. C. Toluidine blue staining of nucleus pulposus cells. D. Immunohistochemically for collagen II of nucleus pulposus cells (Zhang C, Tong T, et al., 2021).
Vitamin D Inhibits TNF-α Induced Apoptosis of Human Nucleus Pulposus Cells Through Regulation of NF-kB Signaling Pathway
Low back pain is a common issue, with lumbar disc degeneration being a major risk factor. Apoptosis of nucleus pulposus cells is a key cause of disc degeneration, and cytokines like TNF-α play a significant role. Vitamin D has been shown to have anti-inflammatory and cell-protective effects. Zhang's team explored whether vitamin D can inhibit TNF-α-induced apoptosis of human nucleus pulposus cells and investigate the underlying mechanism, specifically focusing on the NF-κB signaling pathway.
In the TNF-α-treated group, the apoptosis of nucleus pulposus cells was increased in a dose-dependent manner, compared with the control group. At 100 ng/ml, TNF-α induced more extensive apoptosis (apoptotic index, 19.6%±4.6%) than other concentrations (10 and 30 ng/ml) (Fig. 1). As expected, TNF-α treatment significantly increased the apoptosis. However, pretreatment with vitamin D partially reversed TNF-α-induced apoptosis (P<0.05, Fig. 2A). Western blot analysis revealed that TNF-α upregulated cleaved caspase-3 and downregulated Bcl-2 compared with the control group. In addition, these changes were partially reversed by pretreatment with vitamin D (Fig. 2B). Furthermore, the expression of p-p65 induced by TNF-α was also partially blocked by vitamin D (Fig. 2B). No significant difference was found in p65 expression among the three groups (Fig. 2B). To verify whether the NF-κB pathway is involved in TNF-α-induced apoptosis of nucleus pulposus cells, sulfasalazine, a NF-κB pathway inhibitor, was used. They found that pretreatment with sulfasalazine partially reduced the apoptosis rate (Fig. 3A) and it was validated by Western blot (Fig. 3B).
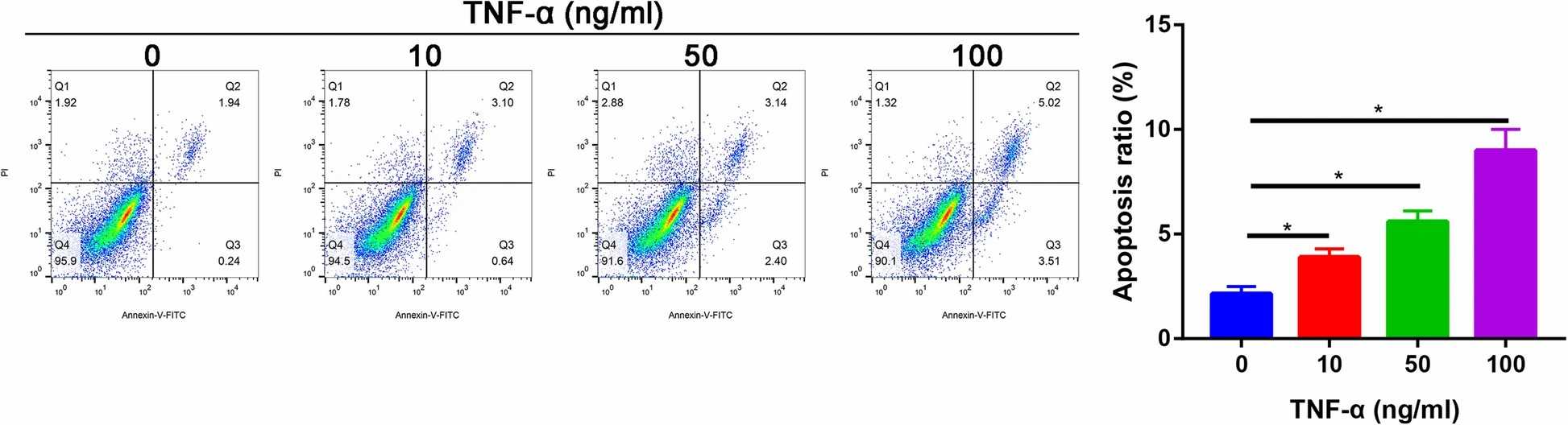 Fig. 1. The apoptotic ratio of nucleus pulposus cells in different concentration of TNF-α groups (Zhang C, Tong T, et al., 2021).
Fig. 1. The apoptotic ratio of nucleus pulposus cells in different concentration of TNF-α groups (Zhang C, Tong T, et al., 2021).
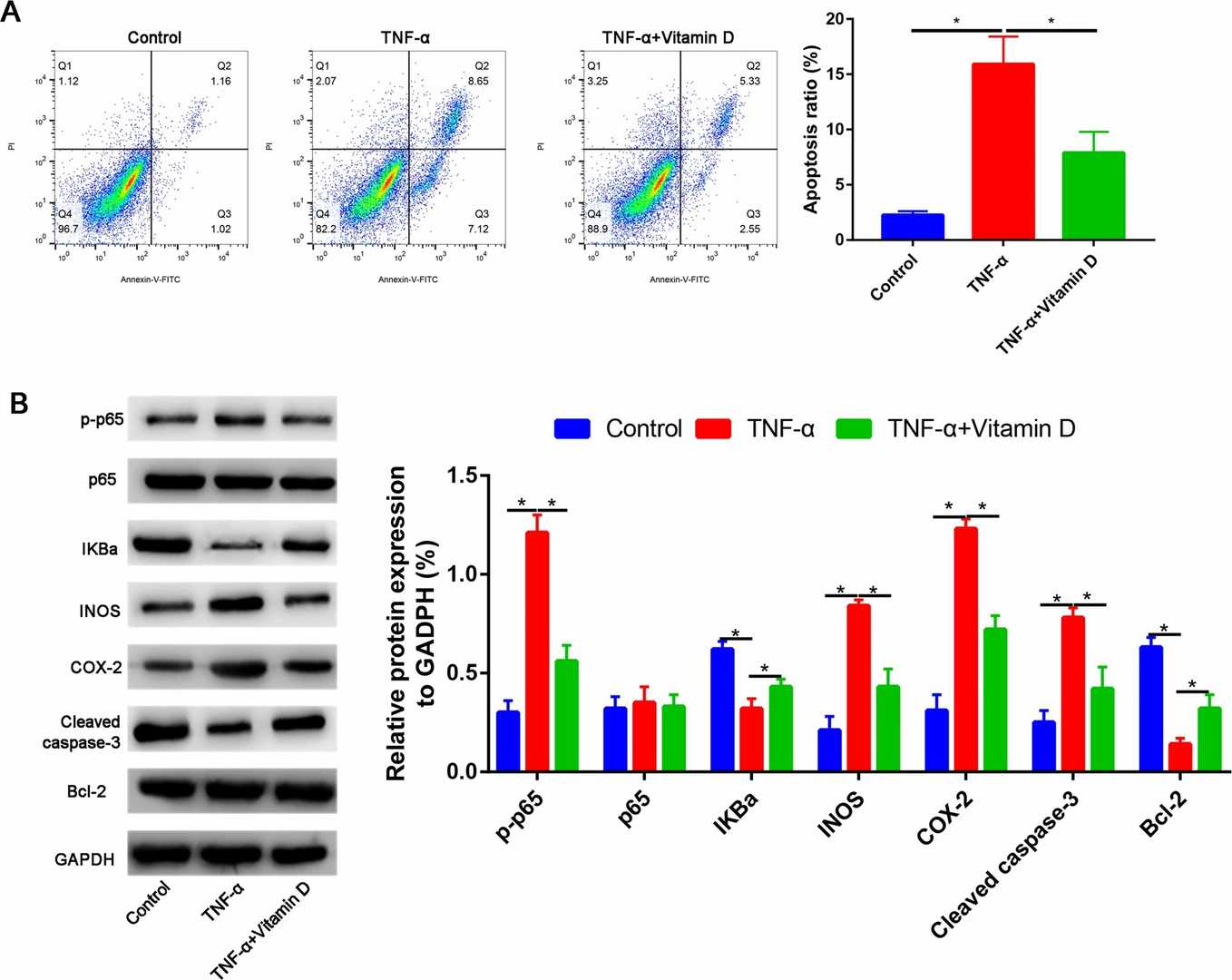 Fig. 2. A Vitamin D partially reversed the TNF-α induced the apoptosis of nucleus pulposus cells. B Western blot assay to assess the Bcl-2, cleaved caspase-3, p-p65, p-65, IKBa, INOS, and COX-2 expressions (Zhang C, Tong T, et al., 2021).
Fig. 2. A Vitamin D partially reversed the TNF-α induced the apoptosis of nucleus pulposus cells. B Western blot assay to assess the Bcl-2, cleaved caspase-3, p-p65, p-65, IKBa, INOS, and COX-2 expressions (Zhang C, Tong T, et al., 2021).
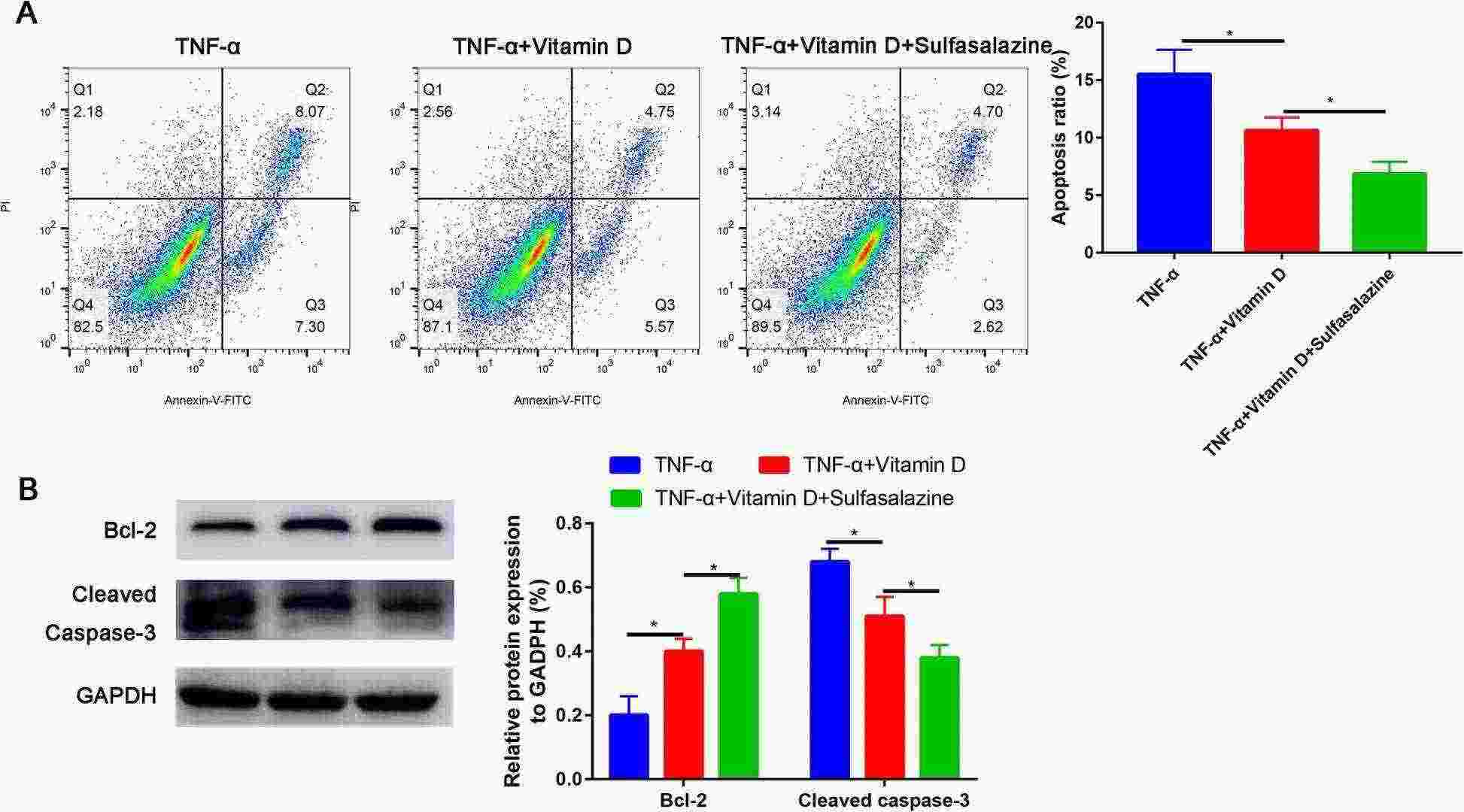 Fig. 3. Sulfasalazine partially recovered the apoptosis rate of TNF-α-treated nucleus pulposus cells (Zhang C, Tong T, et al., 2021).
Fig. 3. Sulfasalazine partially recovered the apoptosis rate of TNF-α-treated nucleus pulposus cells (Zhang C, Tong T, et al., 2021).
UTI Could Enhance the Cell Viability of NP Cells and Promote the Proliferative Ability of NP Cells
Low back pain (LBP) is one of the most common public health concerns, and the degeneration of intervertebral disc (IDD) contributes to LBP. Inflammation and oxidative stress are the principal pathophysiological processes involved in IDD, including cytokines IL-1β and TNF-α. Ulinastatin (UTI) is an endogenous acidic glycoprotein with anti-TNF-α releasing ability and protective effect on the organs under inflammatory damage. However, the potential effect of UTI on human NP cells and its molecular mechanisms remain unclear.
To explore the potential effect of UTI on human NP cells, Luo's group firstly conducted the CCK-8 assay kit to evaluate the cytotoxic effect of UTI on NP cells. As shown in the results, UTI could increase the cell viability of human NP cells without significant cytotoxicity in vitro in a concentration below 4000 U/mL with the IC50 of 6898 U/mL, indicating that the safety range of UTI was wide (Fig. 4a and b). Moreover, Western blot results suggested that UTI could promote the expression of PCNA in NP cells in a concentration-dependent manner (Fig. 4c and d). Immunofluorescence analysis for Ki-67, a proliferation maker, also confirmed the promoting effect of UTI on the proliferation ability of human NP cells (Fig. 4e and f).
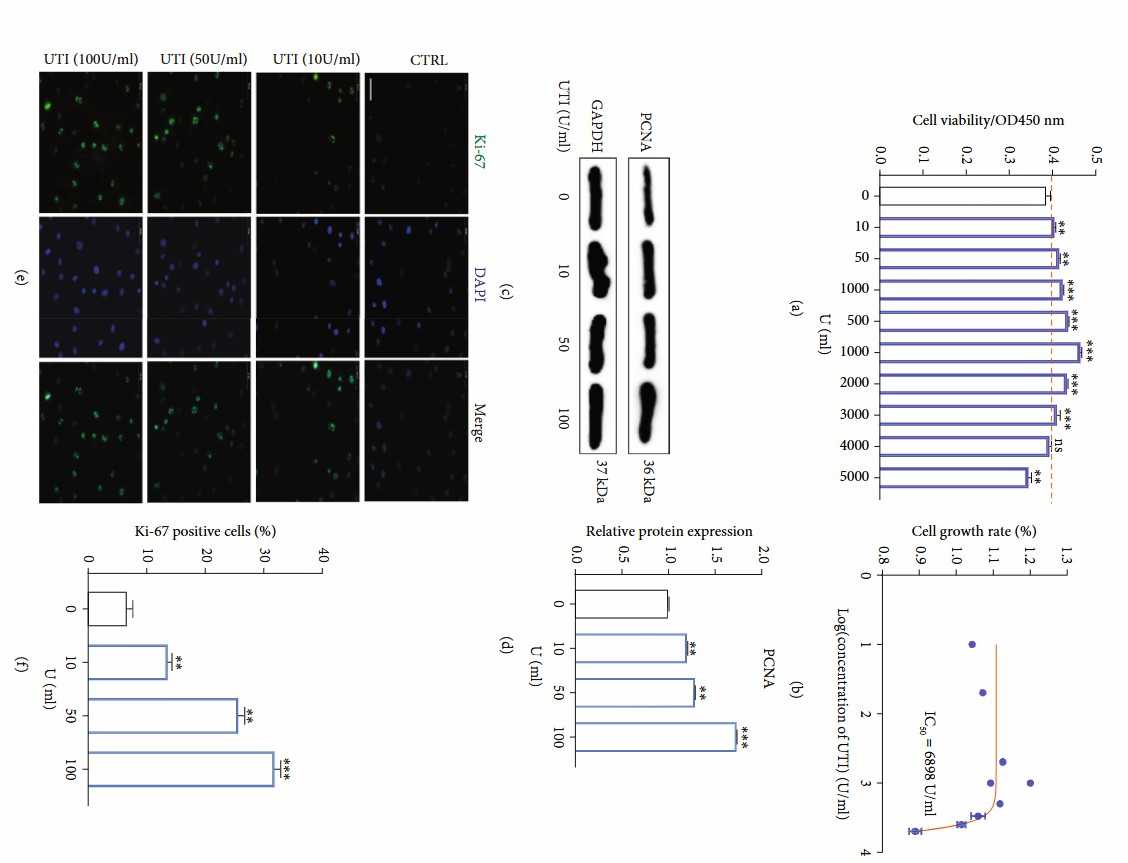 Fig. 4. Effects of UTI on the proliferative ability in human NP cells (Luo X, Huan L, et al., 2021).
Fig. 4. Effects of UTI on the proliferative ability in human NP cells (Luo X, Huan L, et al., 2021).
Ask a Question
Write your own review