Primary Human Vascular Smooth Muscle Cells
Cat.No.: CSC-C4357X
Species: Human
Source: Umbilical Cord; Artery
Cell Type: Smooth Muscle Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
These cells were originated using CSCM Complete Medium, are available at <12 Cumulative Population Doublings (CPD) in vitro [Passage 3] and were cryopreserved in aliquots of ~1.5 X 10^6 cells. This vial will initiate a Passage 4 cell culture in a 75cm2 flask.
These cells are available in both cryopreserved vials as well as in 25cm2 and 75cm2 proliferating culture flasks.
Each vial or flask of cells is shipped to Customer with Bac-O (antibiotic) and SuperEnergy™ (animal derived growth factors) or SuperEnergy-R™ (human recombinant growth factors) at no additional cost.
Primary Human Vascular Smooth Muscle Cells (hVSMCs) are primary cells derived from the tunica media of human blood vessels (ascending/descending aorta, coronary artery, pulmonary artery). They are isolated by enzymatic digestion of fresh vascular tissue, then typically cryopreserved at low passage (P1 or P2) to ensure high viability and plating efficiency. hVSMCs characteristically express the contractile‑type proteins α‑smooth muscle actin (α‑SMA), SM22α (transgelin) and calponin, which are routinely used to confirm their smooth‑muscle identity. Early passage cells (P2‑P4) have a spindle‑shaped, "hill‑and‑valley" morphology and >90 % α‑SMA positivity, but later passages acquire a synthetic phenotype with reduced expression of these markers.
In vivo hVSMCs regulate vascular tone, extracellular‑matrix production, and vessel remodeling. They can undergo phenotypic switching from a contractile to a proliferative/synthetic state in response to growth factors (PDGF, TGF‑β) or mechanical stress. This is a key event in vascular diseases such as atherosclerosis, hypertension, restenosis and aneurysm formation. Primary hVSMCs are used to model various vascular diseases (atherosclerosis, hypertension, restenosis, aortic aneurysm), screen anti‑proliferative/vasodilatory compounds, and study gene‑editing targets/pathways. Co‑culture with primary endothelial cells permits more physiologically relevant vessel‑wall models for tissue‑engineering and high‑throughput drug screening
.
Development of the BioHybrid Calcification Assay
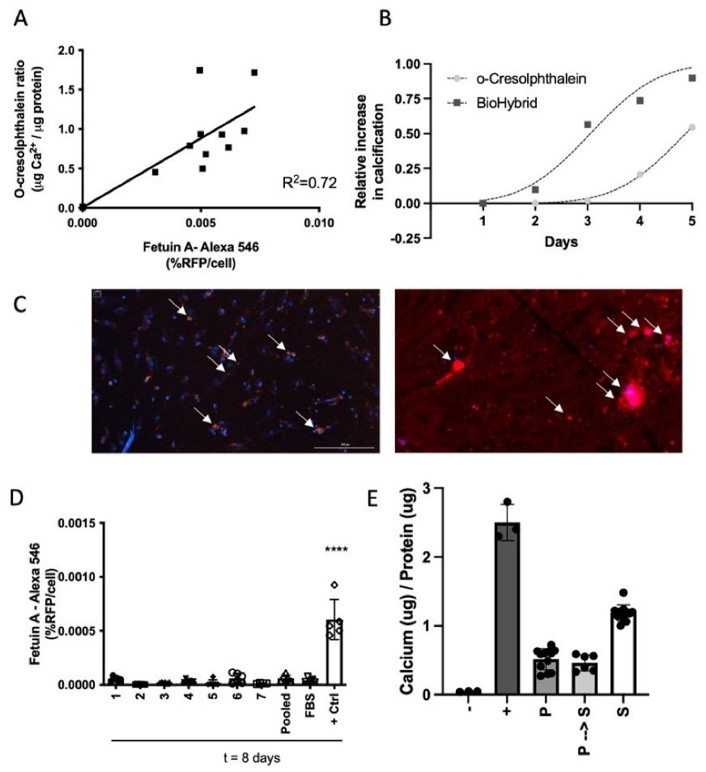
Vascular calcification increases CVD risk, but an appropriate biomarker is lacking. Jaminon et al. developed the BioHybrid assay to measure in vitro calcification in human vascular smooth muscle cells (hVSMCs) and compared it with the o-Cresolphthalein and T50 assays.
They found a strong link between fetuin-A-AlexaFluor®-546 fluorescence and calcium levels in cells (Fig. 1A). The BioHybrid assay is more sensitive and has a better range for detecting early and low levels of calcification compared to the o-Cresolphthalein method (Fig. 1B). A big advantage of using fetuin-A-AlexaFluor®-546 is that it allows real-time monitoring of calcification in human vascular smooth muscle cells (hVSMCs). This method provides sequential, quantifiable measurements of calcification in one assay. They tested different serum and calcium conditions and found that 5% serum or plasma and 3.6 mM calcium chloride (CaCl2) were optimal for robust calcification. They also checked if human serum or plasma behaves similarly in calcification assays. They found no differences in calcification when using fetal bovine serum (FBS) compared to human serum or plasma from healthy controls (Fig. 1D-E). This means the BioHybrid assay can use either serum or plasma samples to measure calcification, and the results are comparable to the o-Cresolphthalein method.
Unbiased Proteomic Analysis of Extracellular Vesicles Secreted by Senescent Human Vascular Smooth Muscle Cells Reveals Their Ability to Modulate Immune Cell Functions
Atherosclerosis is an age-related disease characterized by immunological activity. Atherosclerotic plaques consist of endothelial cells, VSMCs, lipids, and immune cells. VSMCs undergo senescence and secrete SASP factors that modulate the plaque microenvironment. Głuchowska et al. investigated the role of extracellular vesicles (EVs) secreted by senescent VSMCs in modulating the plaque microenvironment and immune cell activity.
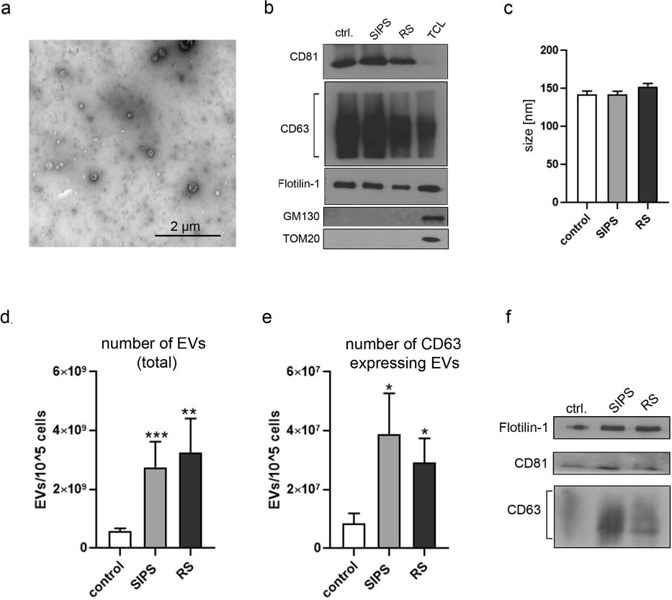
To study the role of EVs from senescent VSMCs, they used two setups: stress-induced premature senescence (SIPS) and replicative senescence (RS). In SIPS, human VSMCs were treated with H2O2 and cultured for 7 days. In RS, cells were cultured until they stopped replicating. SA-β-gal activity and Ki-67 expression confirmed that most cells became senescent. They previously found that senescent VSMCs secrete more SASP factors like IL-6, IL-8, and VEGF. Here, they analyzed the vesicular components of SASP-EVs. EVs from VSMCs were isolated by ultracentrifugation after 24 hours. TEM imaging showed the isolated vesicles (Fig. 2a). Western blot analysis of CD63, CD81, and Flotilin-1 confirmed that the isolated vesicles were exosomes. The purity of EVs was verified by the absence of GM130 and TOM20 markers (Fig. 2b). EV size did not differ between non-senescent and senescent cells (Fig. 2c). However, SIPS and RS senescent VSMCs secreted more EVs than controls (Fig. 2d). ExoElisa and Western blot analyses showed that senEVs contained more exosomes than EVs from control cells (Fig. 2e, f).
Ask a Question
Write your own review