Porcine Aortic Endothelial Cells
Cat.No.: CSC-C1755
Species: Pig
Source: Aorta
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
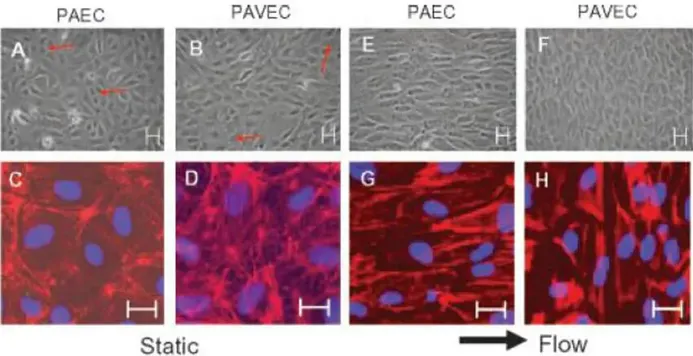
Porcine Aortic Endothelial Cells (PAEC) is a primary cell line harvested from endothelium that lines porcine aorta. PAECs have been shown to be one of the hardiest and most physiologically relevant primary cells to use in vitro. These cells display classical "cobblestone" morphology at confluency and test positive for routine endothelial markers such as Von Willebrand Factor (vWf) and CD31. Due to its similar size and hemodynamics compared to human cardiovascular system, PAEC lines can be considered analogous to human primary endothelial cells and can act as an alternate model that is easier to obtain and more standardized.
Due to their diverse applications in physiological mechanisms PAECs have become essential in studying cellular responses in various processes. Functions of endothelial cells range from maintaining vascular tone to blood-tissue barrier and inflammatory modulation with expression of molecules such as ICAM-1 and VCAM-1. In addition, PAECs are often utilized in examining angiogenesis, nitric oxide production, and shear stress responses. Endothelial cells' ability to create capillary-like structures when induced on basement membrane matrices allows researchers to mimic microenvironments of the vascular wall. PAECs have also become more popular to xenotransplantation applications and testing the biocompatibility of medical devices. Endothelial cells line the entire blood vessel, so PAECs have been used as a method of examining interspecies immunological relationships. Often times these cells are used to test vascular stents and grafts before they are implemented. These cells can be grown in standard endothelial cell media with the addition of VEGF and heparin growth factors.
Human IL-17 and TNF-α Synergistically Regulated the Expression of Various Immune-Related Genes in PAECs
Immune rejection is one of the most critical hurdles for prolonging survival time of porcine xenografts in primates. Although IL-17 and TNF-α are pivotal mediators in several immune disorders, their roles in xenotransplantation have not been well defined. Therefore, Li's team aimed to determine how hIL-17 and hTNF-α regulate gene expression and immune response in PAECs.
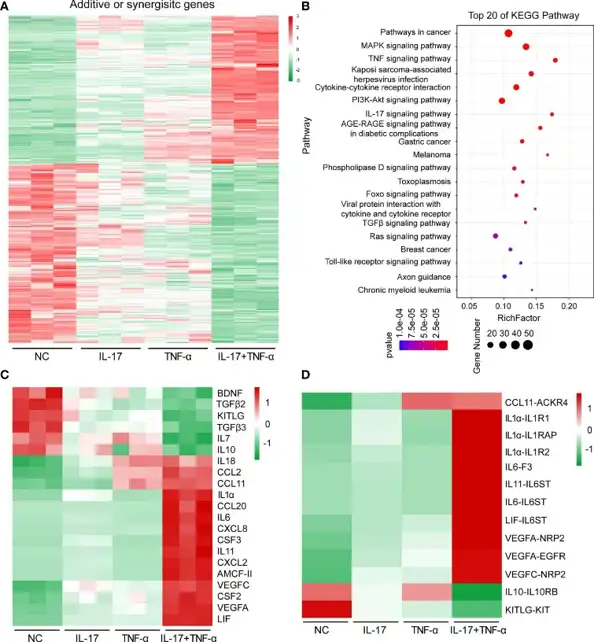
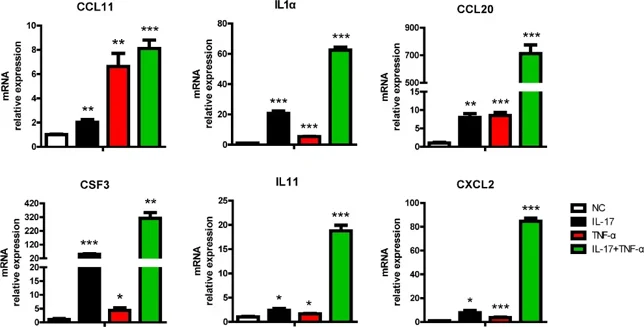
Treatment with human IL-17 (hIL-17) and human TNF-α (hTNF-α) altered the expression of 697 genes in PAECs, of which 315 genes were upregulated (166 synergistically and 149 additively), and 382 genes were downregulated (249 synergistically and 133 additively) (Fig. 1A). Functional annotation of DEGs using KEGG pathway analysis revealed enrichment of immune-associated signaling pathways including TNF-α signaling, IL-17 signaling, MAPK signaling, and Toll-like receptor signaling, cytokine-cytokine receptor interaction, etc. (Fig. 1B). When focused on signal transduction (n = 159), the immune system was found to be the most enriched category involving 86 genes (Fig. 1C). Among them, a series of proinflammatory cytokines (IL1α, IL6) and chemokines (CCL2, CCL11, CXCL8, CXCL2) were induced, while IL10 anti-inflammatory gene was inhibited. Ligand-receptor relationship analysis further identified that CCL11, IL1α, IL6, IL11 and their corresponding receptors were upregulated, whereas IL-10-IL10RB and KITLG-KIT were downregulated (Fig. 1D). RT-PCR validation showed that CCL20, CSF3, IL11, and CXCL2 were synergistically induced by IL-17 combined with TNF-α, whereas CCL11 and IL1α were additively induced by these cytokines (Fig. 2). These data are highly consistent with transcriptome sequencing results, demonstrating that hIL-17 and hTNF-α synergistically upregulated proinflammatory cytokines and chemokines to promote inflammation.
After washing it twice with PBS, add trypsin in, shake it, pour the trypsin out quickly, wash it again with PBS, and add trypsin for normal digestion.
Ask a Question
Average Rating: 4.0 | 1 Scientist has reviewed this product
Good growth
The cells are in good growth condition during the experiment.
09 Dec 2022
Ease of use
After sales services
Value for money
Write your own review