
Human Small Intestinal Fibroblasts
Cat.No.: CSC-C4855L
Species: Human
Source: Small Intestine; Intestine
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
Human Small Intestinal Fibroblasts (HSIFs) are primary stromal cells directly isolated from the lamina propria and submucosal layers of the human small intestine. Morphologically, HSIFs recapitulate the classic spindle-shaped elongated fibroblast phenotype and give rise to the characteristic parallel or swirling monolayers observed for primary fibroblasts in adherent culture. HSIFs express canonical fibroblast markers including vimentin, fibronectin, collagen I/III and also upregulate α-smooth muscle actin upon activation by fibrogenic factors such as TGF-β. Functionally, HSIFs are the major regulators of the intestinal microenvironment, playing critical roles in the synthesis and remodeling of ECM components, epithelial cell proliferation and differentiation via paracrine support, wound repair, and mucosal regeneration. HSIFs are also immunomodulatory cells that communicate with both innate and adaptive immune populations and have a central role in mediating chronic inflammation and intestinal fibrosis.
For these reasons, HSIFs are an attractive and physiologically relevant model for a variety of applications ranging from research of inflammatory bowel disease, epithelial-stromal interactions, intestinal barrier biology, and fibrosis-associated pathways to integration into state-of-the-art in vitro modeling platforms such as gut-on-chip devices and organoid-stromal co-culture systems for recapitulating the structural and functional complexity of the human small intestine. HSIFs are also highly sensitive to a range of cytokines, pathogens, and ECM cues, allowing them to be used for a variety of mechanistic studies as well as preclinical drug screens.
Enhancing Antiviral Immunity in the Gastrointestinal Epithelium: The Role of Fibroblast-Endothelium Interaction and Melatonin
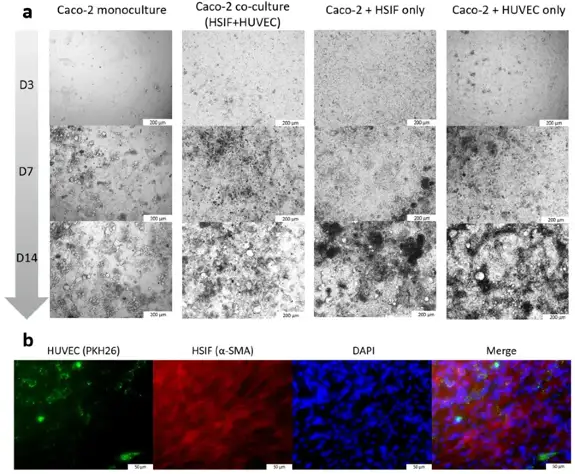
The GI tract is a key barrier against pathogens, but stromal cell contributions to antiviral responses are understudied. Melatonin has antiviral properties, but its GI effects are unclear. Here, Šeškutė et al. stimulated Caco-2 monocultures and co-cultures with human small intestinal fibroblasts (HSIFs) and endothelial cells (HUVECs) using Poly I:C and assessed apoptosis, proliferation, antiviral markers, and organelle activation with and without melatonin.
The co-culture showed slightly faster cell growth (Fig. 1), confirmed by a 32% higher proliferation rate in Caco-2 cells co-cultured with HSIFs and HUVECs (1.68 ± 0.224 vs. 1.13 ± 0.029; p = 0.031). For antiviral response, models were stimulated with Poly I:C. The effect varied by model. Poly I:C induced dose-dependent apoptosis in Caco-2 monoculture and dual co-cultures, reaching 35-40% at 100 μg/mL (Fig. 2a). However, the triple co-culture showed much lower apoptosis, staying below 20% even at high doses (Fig. 2a). This suggests that fibroblasts and endothelial cells protect epithelial cells from virus-mimic-induced apoptosis. Consistent with this, Caco-2 cell viability was better in the triple co-culture (Fig. 2b). High-dose Poly I:C reduced viability to 76-85% in other models, but the triple co-culture remained near control levels (Fig. 2b). These results support the idea that fibroblast and endothelial support enhance epithelial resistance to viral-mimic-induced stress.

Ask a Question
Write your own review
