Rat Primary Cardiac Fibroblasts
Cat.No.: CSC-C4173X
Species: Rat
Source: Heart
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Cells are negative for bacteria, yeast, fungi, and mycoplasma. Rat Primary Cardiac Fibroblasts are tested for expression of marker using the antibody of anti-FSP1/S100A4 by immunofluorescence staining and can be expanded by 2-4 passages at a split ratio of 1:2 under the cell culture conditions specified by Creative Bioarray. Repeated freezing and thawing of cells is not recommended.
Standard biochemical procedures performed with fibroblast cultures include the assays of cell to cell interaction, PCR, Western blotting, immunoprecipitation, immunofluorescent staining, immunofluorescent flow cytometry or generating cell derivatives for desired research applications.
Rat primary cardiac fibroblasts are derived from the interstitium of healthy adult rat heart. They represent about 60‑70% of non‑myocyte cells of heart, and play an important role in homeostasis of extracellular matrix (ECM) and extracellular stiffness, as well as the mediation of cardiac remodeling. Morphologically, the cells exhibit a spindle‑shaped or stellate shape, with 15‑30 µm sized cell body, with a large and lightly stained nucleus. They attach to the culture surface rapidly, and within 24 h form a dense fibrous meshwork. The cells are positive for Vimentin and Fibronectin, and up‑regulate α‑SMA, Collagen I/III, and TGF‑β‑responsive genes upon activation.
The main biological properties of rat cardiac fibroblasts include synthesis of collagen, elastin, and fibronectin, secretion of growth factors such as TGF‑β, PDGF and cytokines (IL‑6, IL‑1β), facilitation of scar formation following myocardial infarction, and involvement in TGF‑β/Smad, Wnt/β‑catenin, MAPK and PI3K/Akt signaling pathways responsible for cardiac fibrosis. These cells are commonly used in in vitro models of fibrosis, gene‑editing experiments, high‑throughput screening of anti‑fibrotic drugs, co‑culture with cardiomyocytes or induced pluripotent stem cell (iPSC)‑derived cells, and mechanotransduction studies (by culturing on stretchable substrates or microfluidic chips).
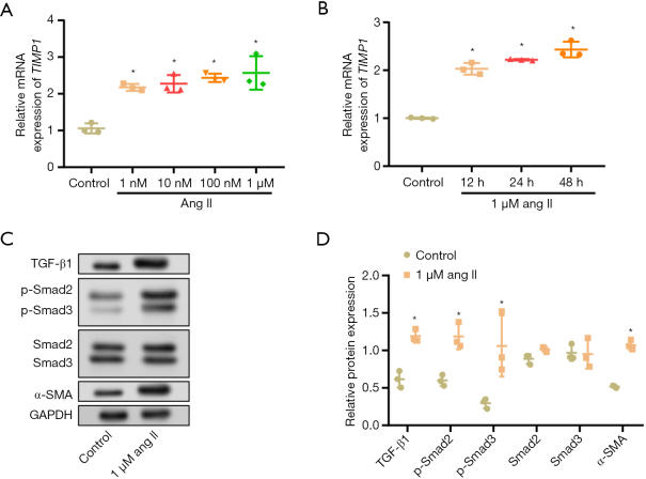
ang II Induced the Upregulation of Rat Cardiac Fibroblasts' Expression of TIMP1 and Activated Fibrotic Signaling
AF is often related to atrial fibrosis, the primary cellular cause of which is cardiac fibroblast-mediated production of ECM. TIMP1, an ECM modulator, has been identified as a critical player in cardiovascular fibrosis; however, its role in AF remains unknown. Zhou's team examined the function of TIMP1 in the angiotensin II (ang II)-induced activation of rat cardiac fibroblasts.
The expression of TIMP1 in rat cardiac fibroblasts treated with different concentrations (1 nM, 10 nM, 100 nM, and 1 µM) and time points (12, 24, and 48 hours) of ang II was analyzed by qRT-PCR assay. The mRNA expression of TIMP1 was found to be maximum on 48 hours of treatment with 1 µM of ang II, which increased in a dose- and time-dependent manner (Fig. 1A, B). Western blotting analysis revealed a marked upregulation of TGF-β1, α-SMA, p-Smad3, and p-Smad2 in cells treated with 1 µM of ang II for 48 hours (Fig. 1C, D). However, non-phosphorylated forms of Smad2 and Smad3 were not significantly altered upon ang II treatment. These results suggest the possible involvement of TIMP1-mediated mechanisms linking ang II stimulation and activation of fibrotic signaling in AF.
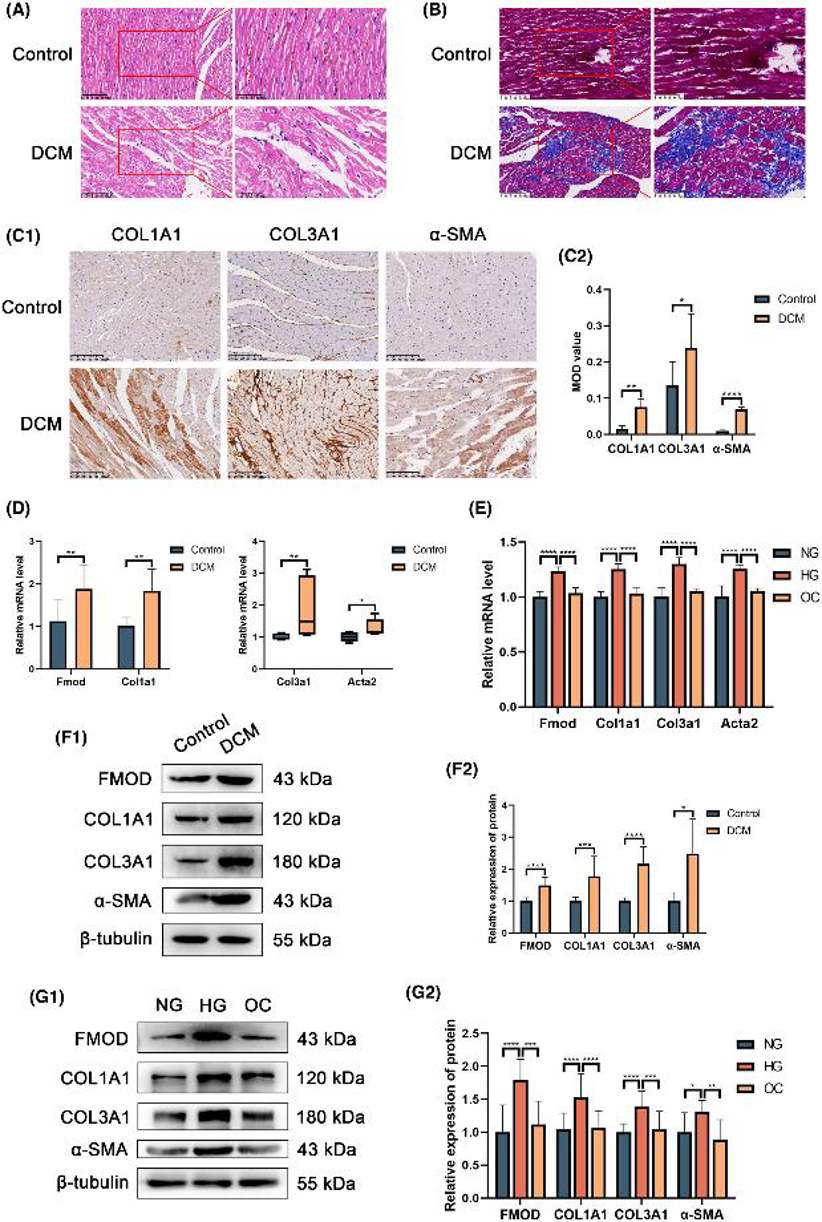
Fmod Increased in DCM Rat Heart and RPCFs Treated with High Glucose of Fibrosis
Diabetic cardiomyopathy (DCM) is marked by excessive extracellular matrix protein deposition, leading to myocardial fibrosis. Fibromodulin (Fmod) is key in fibrotic diseases, but its role in DCM is unclear. Dai's team explored Fmod's role in DCM-related myocardial fibrosis using DCM rat models and high-glucose-exposed rat primary cardiac fibroblasts (RPCFs). They assessed mRNA and protein expression levels of Col1a1, Col3a1, α-SMA and Fmod in both models.
HE staining showed normal arrangement of myocardial fibers and cardiomyocytes in control rat hearts. In contrast, DCM group hearts had irregular, thickened, and fragmented fibers with increased interstitial spacing. Masson trichrome staining revealed more collagen fibers in the DCM group compared to controls. Immunohistochemistry also showed higher levels of Col1a1, Col3a1, and α-SMA in DCM hearts. Primary cardiac fibroblasts had a purity of over 90%. RT-qPCR and Western blot results showed elevated Fmod, Col1a1, Col3a1, and α-SMA levels in DCM hearts and high glucose-treated RPCFs. These findings indicate significant collagen production in DCM rats and enhanced collagen synthesis in RPCFs under high glucose conditions (Fig. 2).
Ask a Question
Write your own review