Human Brain Vascular Smooth Muscle Cells (HBVSMC)
Cat.No.: CSC-7824W
Species: Human
Source: Brain
Cell Type: Smooth Muscle Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human Brain Vascular Smooth Muscle Cells (HBVSMC) are primary‑derived smooth‑muscle cells from the medial layer of human cerebral arteries and micro‑vessels. HBVSMC attach to coated culture surfaces and exhibit a spindle‑to‑epithelial‑like morphology, forming a confluent monolayer in 2-3 days. Immunofluorescence reveals consistent expression of canonical smooth‑muscle markers, such as α‑smooth‑muscle actin (α‑SMA), SM22α, calponin and desmin. In addition, they express adhesion molecules (ICAM‑1 and VCAM‑1) that mediate vascular inflammation. For in vitro culture, HBVSMC are maintained in Smooth‑Muscle Cell Medium (SMCM) or DMEM high‑glucose supplemented with 10 % fetal bovine serum, smooth‑muscle growth supplement, and antibiotics; poly‑L‑lysine coating (2 µg cm⁻²) is needed for optimal attachment.
HBVSMC retain contractile capacity in vitro and can undergo a phenotypic switch to a synthetic, proliferative phenotype in response to cytokines, hypoxia, or mechanical stress. These adaptive processes contribute to cerebrovascular diseases such as atherosclerosis, aneurysm formation, and post‑ischemic remodeling. The expression of ICAM‑1/VCAM‑1 also allows for their use in vascular inflammation studies and blood‑brain barrier integrity models. As a result, HBVSMC are widely used in vitro to: (i) study the mechanisms of cerebrovascular disease; (ii) screen anti‑proliferative or anti‑inflammatory therapeutics; (iii) investigate gene‑editing approaches on smooth‑muscle cell phenotype; and (iv) establish co‑culture blood‑brain barrier models in conjunction with endothelial and pericyte cells. The human origin and brain‑specific context of HBVSMC provide a more physiologically relevant alternative to peripheral vascular smooth‑muscle cell lines.
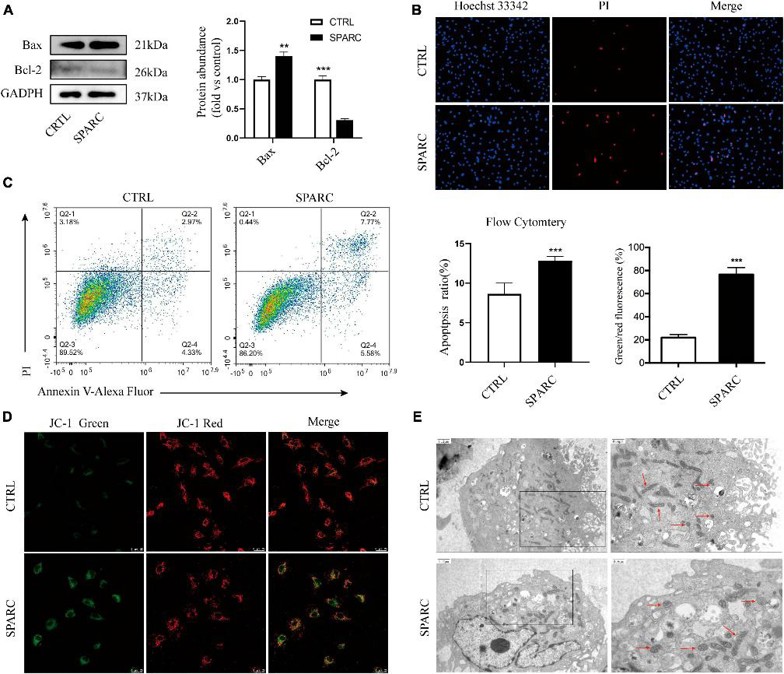
SPARC Induced Apoptotic Cell Death in HBVSMCs
Vascular smooth muscle cell (VSMC) dysfunction is one of the most critical pathologic mechanisms leading to the formation of intracranial aneurysm (IA). Secreted protein acidic and rich in cysteine (SPARC), a multifunctional glycoprotein, is overexpressed in a variety of tumors, but its molecular mechanism in vascular diseases is still unclear. Zhou's team evaluated the potential function of SPARC in IA generation and regulation of mitochondrial function in human brain vascular smooth muscle cells.
Given SPARC's exocrine properties, they previously treated smooth muscle cells with 0-4 µg/mL SPARC for 24 h to assess its effect on cell viability. The CCK-8 assay showed that SPARC inhibited cell viability in a concentration-dependent manner, with significant changes at 2 µg/ml. Thus, 2 µg/mL SPARC was used in subsequent experiments. Western blot showed that SPARC up-regulated the pro-apoptotic Bax and down-regulated the anti-apoptotic Bcl-2 (Fig. 1A). Hoechst 33342/PI staining results showed that the number of blue/red double-stained cells increased slightly in SPARC-treated HBVSMCs, indicating that SPARC induces apoptosis (Fig. 1B). Annexin V/PI flow cytometry also showed that SPARC induced apoptosis and that the apoptotic cells were mainly early apoptotic cells (Fig. 1C). JC-1 MMP detection showed that the mitochondrial membrane potential (MMP) of HBVSMCs decreased after SPARC treatment, and the cells shifted from red light to green light, indicating that MMP was decreased and mitochondria were damaged (Fig. 1D). It suggested that there was a new direction for studying the induction of SPARC apoptosis in HBVSMCs through mitochondrial dysfunction.
Ask a Question
Write your own review