Human Bone Marrow Mononuclear Cells
Cat.No.: CSC-C8039L
Species: Human
Source: Bone Marrow
Cell Type: Mononuclear Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mononuclear cells are prepared by centrifugation in a density cell separation medium from bone marrow. Isolating the MNC eliminates the erythrocytes and graulocytes, producing a more stable cell product. MNC can be used directly in hematopoietic assays, as the starting material for isolating CD34+ progenitor cells.
Human Bone Marrow Mononuclear Cells (hBM-MNCs) are a heterogeneous population of primary cells, which are isolated from human bone marrow after density gradient centrifugation and consist of lymphocytes, monocytes, hematopoietic stem and progenitor cells as well as low numbers of mesenchymal stromal cells. Isolated directly from bone marrow aspirates of healthy donors or patients, hBM-MNCs preserve the native physiological complexity of the bone marrow microenvironment and thus serve as a physiologically relevant ex vivo model system. The various populations of hBM-MNCs show the typical morphology of mononuclear cells (large, round nuclei and little cytoplasm) and an adherence behavior that is dependent on the subpopulation.
Functionally, hBM-MNCs are known to be involved in hematopoiesis, immune regulation and tissue repair processes. These primary cells are often used in basic and translational research to study hematopoietic stem cell differentiation, immune cell development and cytokine signaling. Due to their content of hematopoietic progenitors and monocytes (CD34⁺ and CD14⁺, respectively), hBM-MNCs can be used to generate different immune subsets like dendritic cells, macrophages or natural killer cells for immunological assays or drug testing. In regenerative medicine, these primary cells have been used to study angiogenesis and bone regeneration as well as for cellular therapies of ischemic and degenerative disorders. Furthermore, hBM-MNCs have been established as a physiologically relevant platform for toxicology testing, for example of gene therapy vectors or ex vivo expansion protocols.
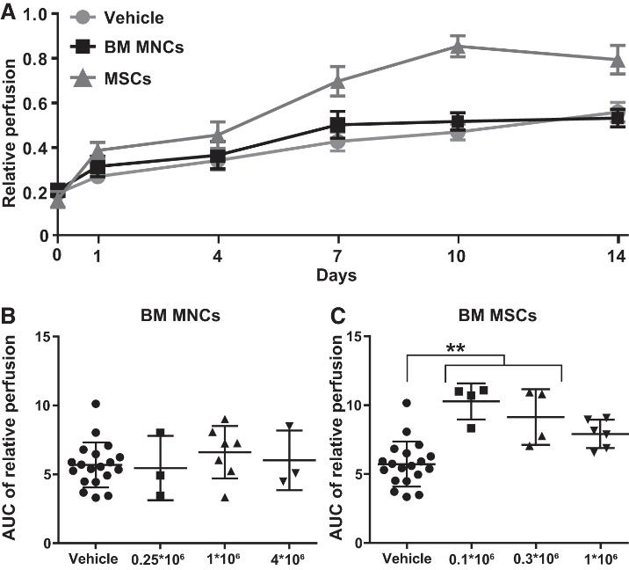
Human Bone Marrow Mononuclear Cells Do Not Improve Limb Perfusion in the Hindlimb Ischemia Model
Effective treatments for chronic limb-threatening ischemia are lacking. Studies on using bone marrow (BM) mononuclear cells (MNCs) and BM-derived mesenchymal stromal cells (MSCs) have shown variable results, and no studies have directly compared human BM MNCs and BM MSCs in in vivo models. van Rhijn-Brouwer et al. studied the effects of intramuscular administration of human BM MNCs and MSCs on limb perfusion in a murine hindlimb ischemia (HLI) model.
On day 0, mice underwent femoral ligation and received intramuscular injections of the prepared cells on day 1. Average relative perfusion was 17% on day 0 and 31% on day 1 (Fig. 1A). No animals were lost during surgery or excluded due to relative perfusion >50% on day 1. There were no adverse events from cell implantation, no limbs were lost, and no premature mortality occurred before the planned end. Thus, no animals reached a humane endpoint. Relative perfusion of the ligated limb versus the control limb is shown in Fig. 1A. Two-way ANOVA with Dunnett correction showed that at day 14, BM MNCs did not improve perfusion over vehicle, while BM MSCs significantly outperformed both BM MNCs and vehicle. For subgroup analysis, they calculated the AUC for each animal. There was no difference between vehicle and the three BM MNC doses (Fig. 1B). For BM MSCs, the 0.1 and 0.3 M groups were significantly better than vehicle (Fig. 1C), while the 1 M group was not, suggesting a nonlinear dose-response relationship with an optimum at the lowest dose.
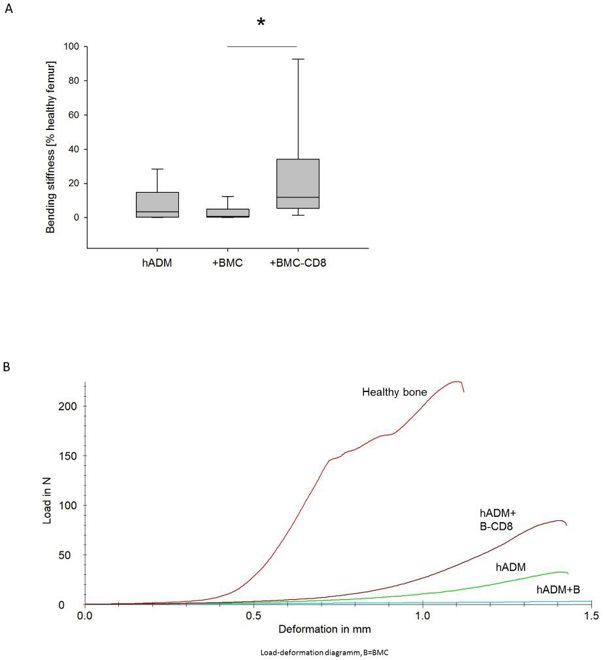
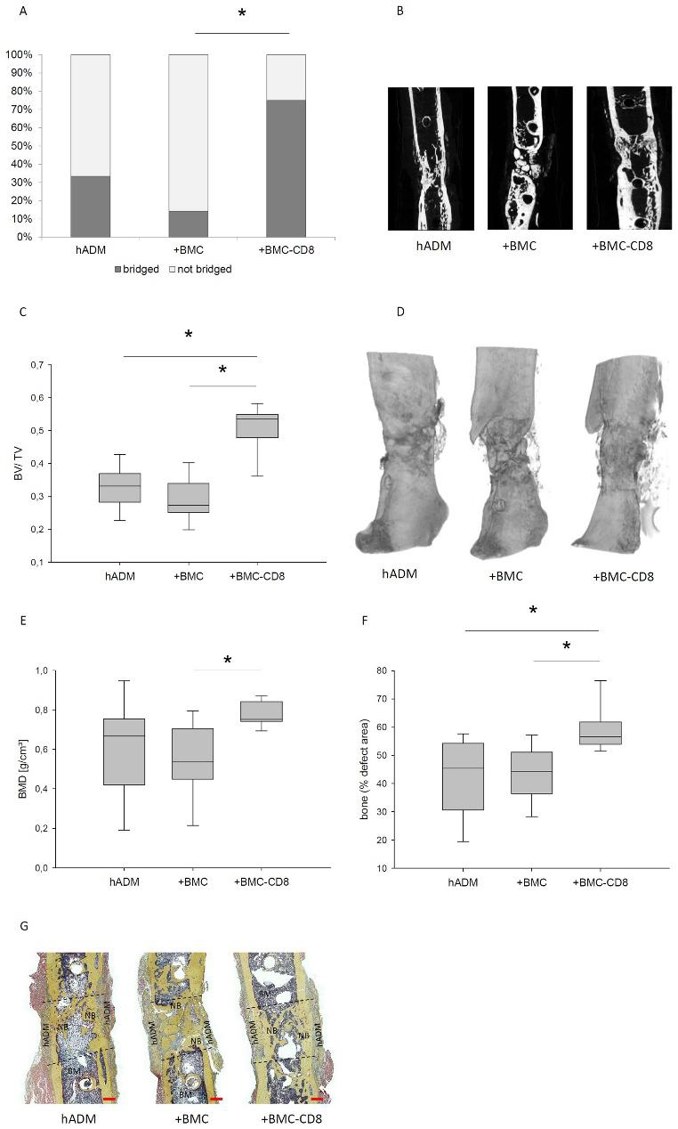
Enhancing One-Step Membrane Technique for Large Bone Defects with CD8-Depleted BMC in Rat Model
The one-step membrane technique using a human acellular dermal matrix (hADM) is an experimental method for treating large bone defects. This technique eliminates the need for the Masquelet membrane induction step, shortening the procedure while maintaining effectiveness. However, previous studies showed that colonizing hADM with bone marrow mononuclear cells (BMC) worsens healing, likely due to the presence of CD8+ lymphocytes, which negatively affect bone regeneration. Pemma-Martinez et al. investigate whether the negative impact of BMC on bone healing is due to the CD8+ cell population.
The bending stiffness of the defect zone was measured 8 weeks after grafting using a three-point bending test. Animals receiving hADM+BMC-CD8 had a notable increase in femoral bending stiffness (median: 11.7%; IQR 5.2-44.5) compared to the hADM+BMC group (median: 0.8%; IQR 0.17-6.7), but not compared to the hADM group (median: 3.3%; IQR 0.3-23.6) (Fig. 2). Defect bridging, callus volume, and bone mineral density were selected as relevant factors to assess bone healing. These parameters were obtained from µCT data. Implantation of hADM+BMC-CD8 resulted in a significantly increased ratio of osseous-bridged bone defects compared to hADM+BMC, but not compared to hADM. An increase in the BV/TV ratio was observed in animals transplanted with hADM+BMC-CD8 compared to hADM and hADM+BMC. The hADM+BMC-CD8 group had significantly higher BMD than the hADM+BMC+CD8 group (median 0.54 g/cm³, IQR 0.42-0.73), but the comparison with the hADM group was not significant (p = 0.14). Radiological findings were generally confirmed by bone histology. Analysis of Movat's pentachrome stained histology slides showed that bone formation was more pronounced in defects treated with hADM+BMC-CD8 (56%, IQR 53-62) compared to hADM+BMC (44%, IQR 36-52) and hADM (45%, IQR 30-54) (Fig. 3).
Creative Bioarray's mononuclear cells are isolated from bone marrow, cord blood, or peripheral blood via density gradient centrifugation.
No, the cells will proliferate and differentiate simultaneously upon being placed in culture. Varying the cytokine cocktail can influence the differentiation and proliferation of the cells.
Used for a variety of applications, BMMCs are frequently utilized in drug discovery/development, assay validation/development, cell therapy research, and other immunology applications.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
High purity
High-purity bone marrow mononuclear cells.
10 May 2023
Ease of use
After sales services
Value for money
Write your own review
- You May Also Need