Human Placental Microvascular Endothelial Cells
Cat.No.: CSC-C4865L
Species: Human
Source: Placenta
Cell Type: Endothelial Cell; Microvascular Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
Human placental microvascular endothelial cells (HPMEC) are primary endothelial cells harvested from villous microvasculature of term human placenta. Their strong expression of von Willebrand factor (vWF), CD31 and VE‑cadherin, and lack of hematopoietic (CD45) and smooth‑muscle markers have been confirmed by immunofluorescence, and supports a pure endothelial phenotype.
In vitro, HPMEC can be maintained in endothelial cell medium (ECM) supplemented with growth factors. They can be passaged for approximately 10 population doublings before becoming senescent, and are known to retain functional characteristics of cultured endothelial cells, including Dil‑Ac‑LDL uptake, Matrigel tube formation, and a robust proliferative response to VEGF and fibroblast‑growth factor‑2. In vivo, HPMEC are the cellular components of the fetal side of the placental barrier. As endothelial cells, HPMEC have been shown to regulate vascular permeability and secrete angiogenic factors (VEGF, PIGF) and adhesion molecules (ICAM‑1, VCAM‑1) in response to inflammatory stimuli, including TNF‑α or hypoxia. The exosomes released by HPMEC contain microRNAs (e.g. miR‑486‑5p) that regulate trophoblast migration, and have been suggested to play a role in the pathophysiology of pre‑eclampsia.
Owing to their ability to accurately recapitulate the human placental microvascular environment, HPMEC have been employed in a variety of contexts, most commonly as in vitro models of maternal‑fetal exchange, drug‑transfer studies, and to help investigate the mechanisms of placental vascular disorders.
Exosomal miR-486-5p Derived from Human Placental Microvascular Endothelial Cells Regulates Proliferation and Invasion of Trophoblasts
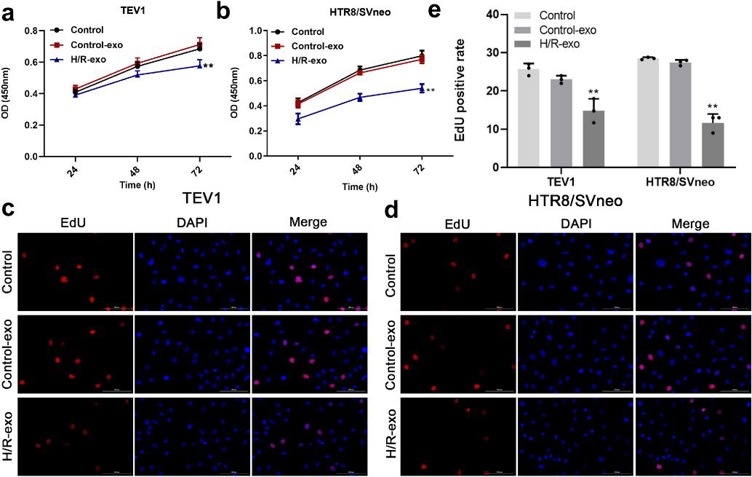
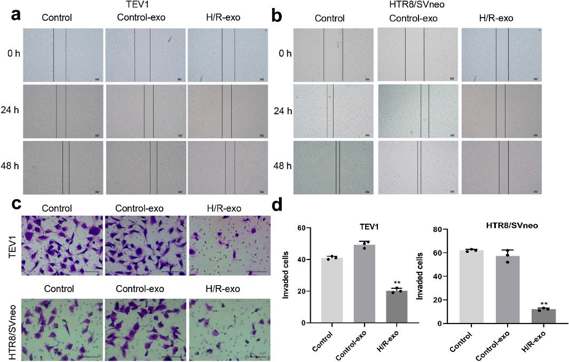
Preeclampsia (PE) is a serious pregnancy complication with upregulated exosomes. Here, Ma's team treated human placental microvascular endothelial cell (HPVEC) with hypoxia/reoxygenation (H/R) and examined the effects of exosomal miR-486-5p on trophoblast cells. To investigate the effect of exosomes on trophoblast cell proliferation, a CCK-8 assay was conducted. The results showed that exosomes from H/R-induced HPVECs significantly decreased the viability of TEV1 or HTR8/SVNEO cells (Fig. 1a, b) and reduced the EdU staining positive rate in trophoblast cells (Fig. 1c-e). In summary, exosomes from H/R-induced HPVECs significantly inhibited trophoblast cell proliferation. To assess the impact of exosomes from H/R-induced HPVECs on trophoblast cell migration, wound-healing assays were performed. As shown in Fig. 2a, b, these exosomes notably decreased the migration of TEV1 or HTR8/SVNEO cells, and a transwell assay indicated reduced invasion of trophoblast cells (Fig. 2c, d). Overall, exosomes from H/R-induced HPVECs significantly decreased trophoblast cell migration and invasion.
Ask a Question
Write your own review


