Rat Pulmonary Artery Endothelial Cells
Cat.No.: CSC-C4139X
Species: Rat
Source: Lung; Artery
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Rat Pulmonary Artery Endothelial Cells (RPAECs) are a primary endothelial cell line originally isolated from the intimal layer of the main pulmonary artery in rats. RPAECs have been described to maintain key features of the endothelial cell phenotype, including the typical cobblestone morphology, robust adhesion to extracellular matrix coated substrates, and the expression of endothelial markers PECAM-1, VE-cadherin, vWF, and eNOS. Endothelial cells in culture, such as RPAECs, are responsible for the active secretion of vasoactive mediators, including nitric oxide, prostacyclin, and endothelin-1, and can be used to accurately model vascular tone and endothelial dysfunction in vitro.
As such, RPAECs demonstrate a phenotypic and functional response to inflammatory cytokines and hypoxic stress, exhibiting rapid changes in permeability, adhesion molecule expression, and oxidative signaling pathways. They have therefore been used extensively in research modeling pulmonary artery hypertension (PAH), endothelial barrier function, leukocyte-endothelial cell interactions, and vascular remodeling. RPAECs are also used as a model system to assess the efficacy of drugs that aim to mitigate endothelial injury, oxidative stress, coagulation, and angiogenic pathways. Owing to their close physiological relevance to in vivo processes and a high responsivity to external stimuli, RPAECs have been used in cardiovascular, pulmonary vascular, inflammatory, and toxicological research studies.
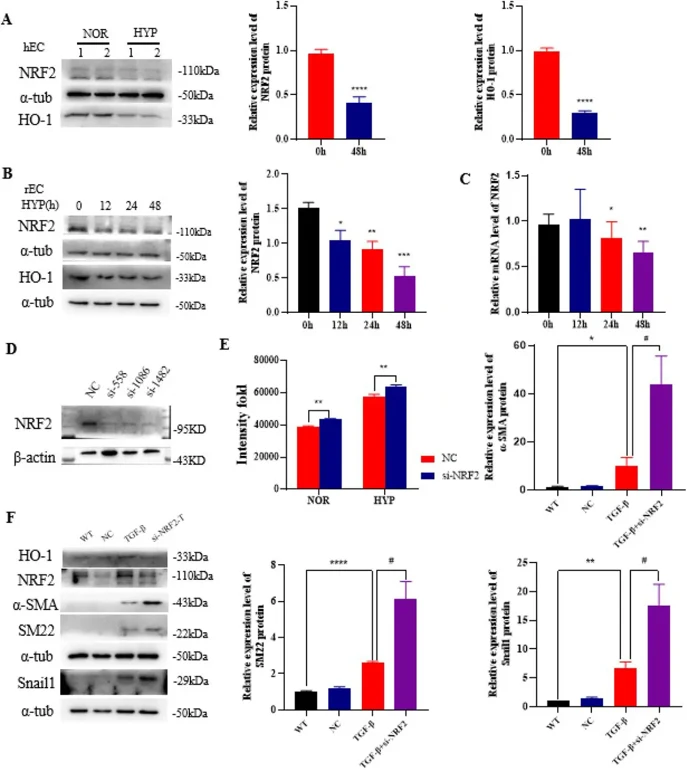
The Effect of NRF2 on EndMT of Rat Pulmonary Artery Endothelial Cells
Pulmonary hypertension (PH) is a fatal disease with no existing drugs that can reverse it. NF-E2-related factor 2 (NRF2) is a key molecule in cell protection. Ning's team examined NRF2 expression in PH models and its role in regulating abnormal phenotypes in pulmonary artery cells.
During pulmonary hypertension, pulmonary vascular cells experience hypoxia, which activates the hypoxia-inducible factor and triggers downstream reactions. To explore hypoxia's effect on NRF2 expression in PAECs, they detected NRF2 and HO-1 protein levels by Western blot in ECs treated with hypoxia. Results showed that NRF2 and HO-1 levels were downregulated in hPAEC and rPAEC under hypoxia (Fig. 1A-B), and NRF2 mRNA expression decreased in hypoxic rPAEC (Fig. 1C). Knocking down NRF2 and treating with TGF-β1 significantly increases mesenchymal markers, suggesting NRF2 may alleviate EndMT (Fig. 1F). Mechanistically, NRF2 knockdown increases ROS levels (Fig. 1E) and Snail1 protein levels in PAECs (Fig. 1F). Thus, NRF2 may regulate EndMT by influencing Snail1 protein levels and ROS levels in PAECs.
Ask a Question
Write your own review