Rat Aortic Endothelial Cells
Cat.No.: CSC-C1976
Species: Rat
Source: Aorta
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Rat Aortic Endothelial Cells, or RAECs, are primary cells that have been harvested from endothelium of the rat aorta. They make up endothelium, a thin semi-permeable membrane, that lines the interior surface of blood vessels. Endothelial cells act as a barrier between blood in your vessels and other cells that make up blood vessels. Endothelial cells lining the vasculature help facilitate homeostasis of blood flow, leukocyte extravasation, and blood vessel contraction and relaxation through the secretion of nitric oxide and endothelin-1. In culture, RAECs will develop the typical "cobblestone" appearance of endothelial cells when confluent. Rat Aortic Endothelial Cells express endothelial cell markers such as CD31(PECAM-1) and von Willebrand Factor. RAEC's have been used as an in vitro model to study various vascular diseases because they are readily accessible and easy to work with. They have been used to study early stages of atherosclerosis as well as angiogenesis, hypertension, and wound healing.
α-Cyperone Alleviates LPS-Induced Pyroptosis in Rat Aortic Endothelial Cells via the PI3K/AKT Signaling Pathway
Pyroptosis is a form of programmed cell death linked to inflammation. Here, Liu et al. used molecular docking to predict α-cyperone's binding affinity to pyroptosis-related proteins and established a pyroptosis model in RAECs using LPS-containing rat serum.
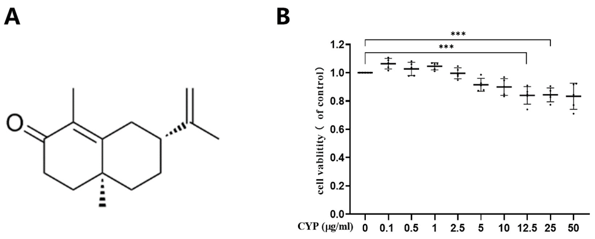
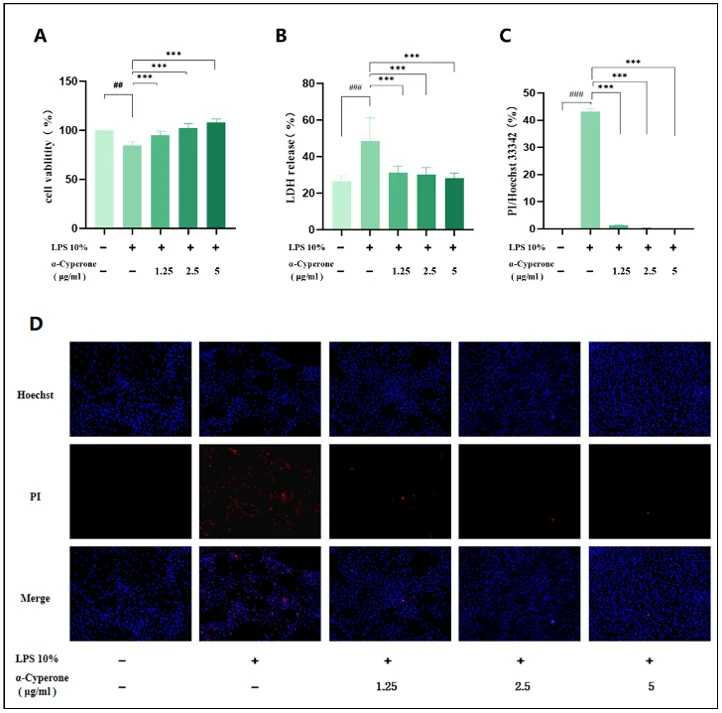
The cytotoxicity of α-cyperone in RAEC was determined by CCK-8 assay after incubation with different concentrations of α-cyperone for 18 h. As displayed in Fig. 1B, α-cyperone was not cytotoxic at the tested concentrations up to 10 μg/mL. To determine the effect of α-cyperone on LPS-induced RAEC death, cell viability was detected using CCK-8 assay. As expected, the viability of cells in LPS group was much lower than that in blank group (Fig. 2A). After α-cyperone pretreatment, cell viability was significantly recovered. LDH release assay and Hoechst 33342/PI double staining assay were applied to determine cell membrane integrity. The results showed that LDH release rate was significantly increased in LPS group compared with controls (Fig. 2B). Pretreatment with α-cyperone dramatically decreased the LDH release rate. Hoechst 33342/PI double staining assay showed that there were more PI-positive(red) (damaged membrane) cells in LPS group (Fig. 2C). Moreover, after pretreatment with α-cyperone, the PI-positive rate was significantly decreased, which was consistent with the LDH release assay results, indicating α-cyperone could alleviate LPS-induced membrane damage in RAEC.
Ask a Question
Write your own review