Human Pulmonary Artery Adventitia Fibroblasts (HPAAF)
Cat.No.: CSC-7730W
Species: Human
Source: Lung; Artery
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human pulmonary artery adventitial fibroblasts (HPAAFs) are fibroblastic cells isolated from the adventitia, the outermost connective tissue layer of the human pulmonary artery. HPAAFs are important structural and regulatory components of the vascular wall. They provide mechanical support to the vessel, contribute to extracellular matrix (ECM) homeostasis, and play a role in coordinating immune-vascular interactions. In vitro, HPAAFs display a spindle-shaped or stellate morphology, form characteristic parallel bundles or whorl-like cell arrangements, and respond robustly to various stimuli, including PDGF-BB, endothelin-1, hypoxia, and inflammatory cytokines. Phenotypically, HPAAFs express vimentin, fibronectin, and FSP1. HPAAFs can also acquire a myofibroblast-like phenotype, characterized by the expression of α-SMA and increased collagen production, under certain pathological conditions.
HPAAFs are involved in pulmonary vascular remodeling processes due to their ability to synthesize and remodel ECM components, regulate MMP/TIMP balance, and modulate inflammatory signaling pathways. They secrete various cytokines, such as IL-6, CXCL12, and MCP-1, which promote immune cell recruitment and contribute to the chronic inflammatory environment observed in pulmonary hypertension and vascular fibrosis. HPAAFs also exhibit sensitivity to hypoxia and mechanical stress, contributing to adventitial thickening and increased vascular stiffness. They are commonly used as an in vitro model for pulmonary arterial hypertension (PAH), vascular inflammation, fibroblast activation, and ECM remodeling. These cells are utilized in drug discovery and antifibrotic screening, cytokine signaling studies, and the generation of advanced vascular models, including multi-layered arterial constructs and vessel-on-chip systems.
Hypoxia Drives Human Pulmonary Artery Adventitia Fibroblasts Collagen Accumulation by Reducing Turnover
HPH is linked to low oxygen levels causing PA narrowing and death. Hypoxia increases PA collagen and alters PAAF metabolism, but the mechanisms are unclear. Here, Philip et al. cultured human pulmonary artery adventitia fibroblasts (HPAAF) in normoxic and hypoxic conditions and treated them with a HIF-1α inhibitor.
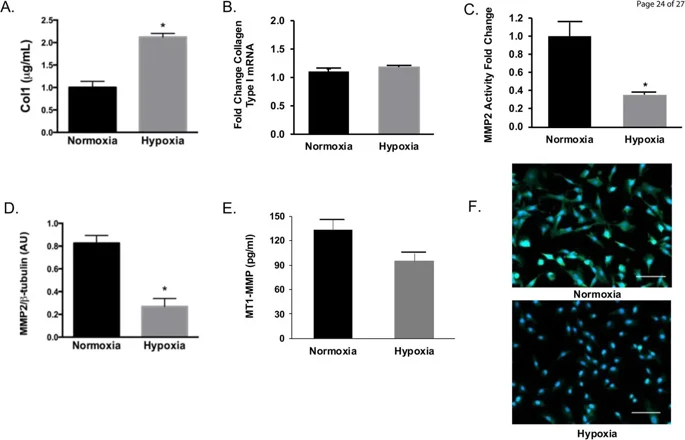
In chronic HPH rodent models, hypoxia leads to collagen accumulation in pulmonary arteries, with collagen type I being the main fibrillar collagen affecting arterial stiffness. Figure 1A shows increased Collagen 1 content in HPAAF under hypoxia, while Collagen 1 mRNA levels remained unchanged (Fig. 1B), indicating that the collagen increase was not due to increased synthesis. MMP activity, crucial for collagen degradation, was significantly reduced in hypoxic HPAAF (Fig. 1C), with MMP2 expression dramatically decreased (Fig. 1D). MT1-MMP concentration was reduced but not significantly different from normoxic conditions (Fig. 1E). Additionally, αSMA expression, indicating myofibroblastic differentiation, was reduced under hypoxia (Fig. 1F). These findings suggest that collagen accumulation in HPAAF under hypoxia is due to reduced degradation rather than increased production.
Ask a Question
Write your own review