BALB/c Mouse Cardiac Endothelial Cells
Cat.No.: CSC-C4345X
Species: Mouse
Source: Heart
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse Cardiac Endothelial Cells can be used in assays of cell to cell adhesion, migration, vascular tube formation, or transendothelial resistance (TER). Standard biochemical procedures performed with endothelial cell cultures include RT-PCR, Western blotting, immunoprecipitation, immunofluorescent staining or immunofluorescent flow cytometry or generating cell derivatives for desired research applications.
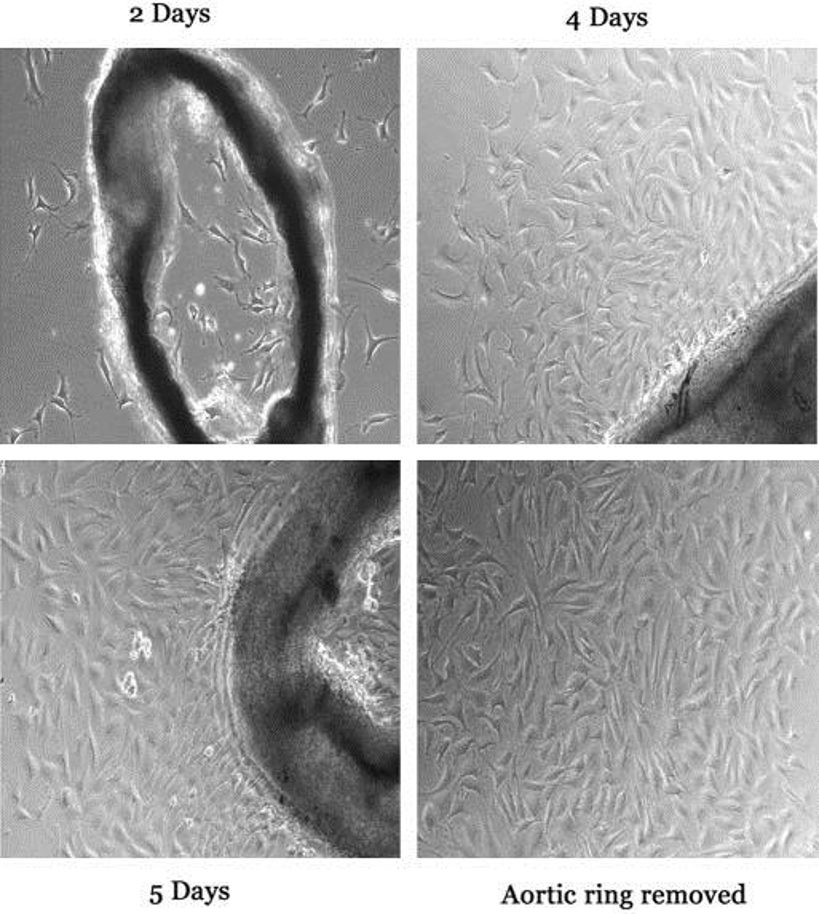
BALB/c mouse cardiac endothelial cell (CEC) is an in vitro endothelial cell line that was isolated from the microvascular and coronary endothelium of adult wild‑type BALB/c laboratory mice. CECs were obtained directly from heart tissue, and they line the inner surface of cardiac capillaries and arteries, where they mediate nutrient, gas, and signal molecule exchange between the blood and the myocardium. They are adherent cells that form a typical "cobblestone" monolayer on a culture substrate. They typically have a diameter of 10-15 µm, a round to oval nucleus, and they express canonical endothelial markers including CD31 (PECAM‑1), VE‑cadherin, and von Willebrand factor. As such, the cells are routinely >90 % pure as determined by immunofluorescence or flow cytometry. CECs require standard endothelial growth media and supplementation with serum, growth factors, and extracellular matrix coatings like gelatin, collagen or poly‑L‑lysine for successful in vitro culture.
Functionally, CECs have strong barrier function as determined by transepithelial electrical resistance measurements, form capillary‑like tubes in Matrigel or collagen-based angiogenesis assays, and will up‑regulate expression of cell adhesion proteins, including ICAM‑1 and VCAM‑1, upon exposure to inflammatory cytokines such as TNF‑α and IL‑1β. As CECs are highly sensitive to important cardiovascular regulators and pathways, including VEGF, FGF, Notch, and Wnt, they can be used to study processes such as angiogenesis, endothelial dysfunction, and myocardial ischemia‑reperfusion injury.
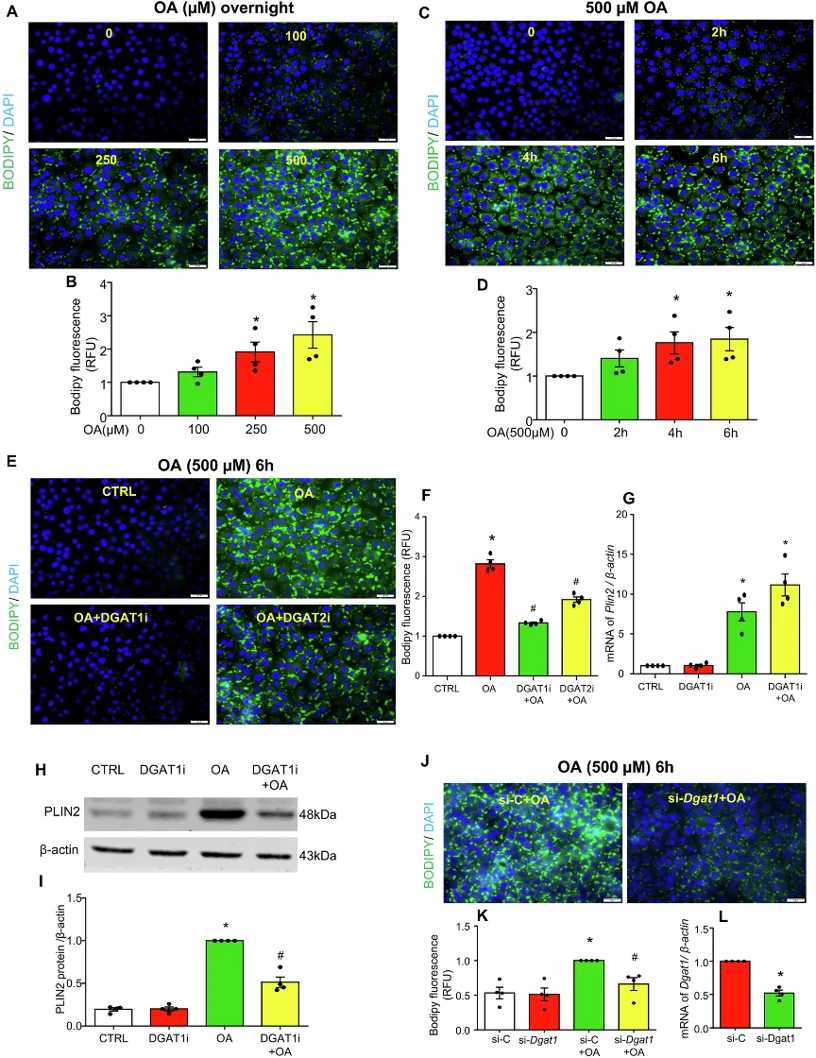
Oleic Acid Induces LD Biogenesis in MCECs
Endothelial dysfunction fuels coronary microvascular disease, yet how lipid droplets (LDs) influence lipotoxic injury in mouse cardiac endothelial cells (ECs) remains elusive. Wang's team tested whether DGAT1-driven LD formation protects mouse cardiac ECs from oleic acid-triggered ferroptosis and mitochondrial failure.
To visualize oleic acid-induced LDs, Bodipy staining was performed in cultured MCECs. It was found that oleic acid dose- and time-dependently increased LD formation as evidenced by increased Bodipy fluorescence (Fig. 1A-D). DGAT1 inhibitor A-922500 abolished this response, whereas DGAT2 inhibitor PF-06424439 only blunted it (Fig. 1E, F). DGAT1i did not prevent oleic acid-induced Plin2 mRNA rise (Fig. 1G) yet lowered PLIN2 protein (Fig. 1H, I), implying transcriptional compensation when LD assembly is blocked. Dgat1 silencing phenocopied the inhibitor (Fig. 1J-L), confirming that oleic acid drives LD biogenesis chiefly via DGAT1 in MCECs.
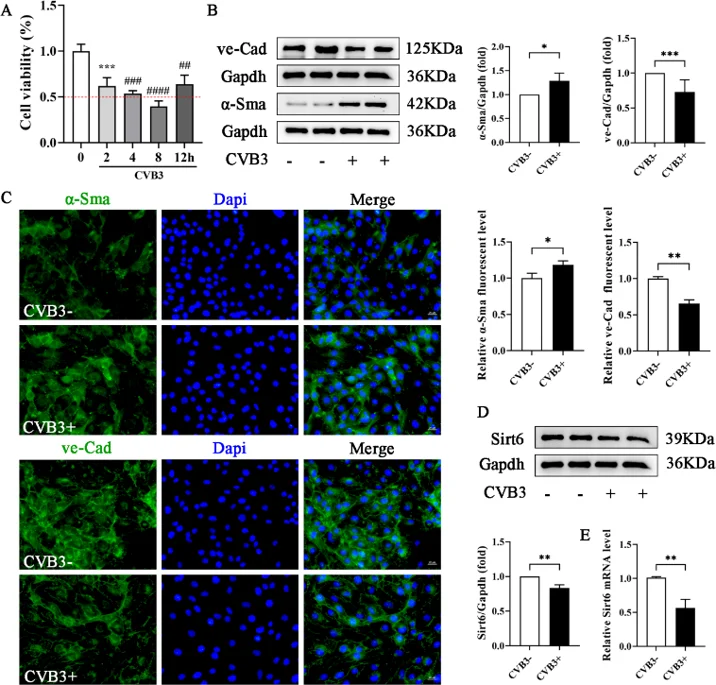
Expression of Sirt6 during the Process of CVB3-Induced EndMT in MCECs
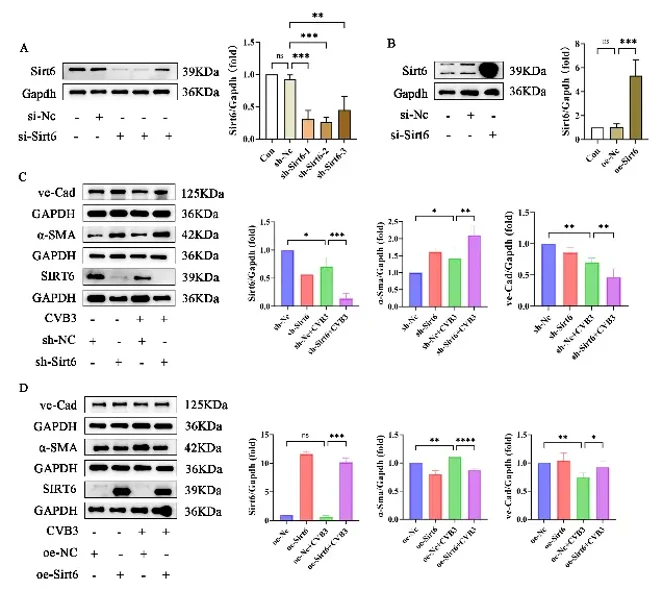
Sirt6 is cardio-protective, but its role in CVB3-driven EndMT is unknown. Yang's team infected MCECs with CVB3, modulate Sirt6 by lentivirus, quantify endothelial/mesenchymal markers, apoptosis, oxidative stress and autophagy via flow cytometry, WB and proteomics. They aimed to establish that Sirt6 over-expression curbs EndMT and apoptosis by dampening oxidative stress and restoring autophagy in CVB3-injured endothelium.
Optimal CVB3 exposure time for MCECs was first determined by CCK-8; 2 h was selected (Fig. 2A). Western blot showed that CVB3 reduced the endothelial marker VE-cadherin and raised the mesenchymal marker α-SMA (Fig. 2B), and immunofluorescence confirmed EndMT (Fig. 2C). CVB3 also lowered SIRT6 at both protein and mRNA levels (Fig. 2D-E). To further verify the impact of Sirt6 on CVB3-induced EndMT, Sirt6 was silenced or over-expressed by lentivirus transfection. Lentiviral knockdown or over-expression of Sirt6 (Fig. 3A-B) revealed that loss of Sirt6 worsened CVB3-induced EndMT (greater α-SMA, lower VE-cadherin; Fig. 3C), whereas Sirt6 over-expression partially preserved endothelial identity by restoring VE-cadherin and suppressing α-SMA (Fig. 3D).
Ask a Question
Write your own review