Rabbit Chondrocytes
Cat.No.: CSC-C5284S
Species: Rabbit
Source: Cartilage
Cell Type: Chondrocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Rabbit chondrocytes from Creative Bioarray are isolated from the rabbit joint tissue. The method we use to isolate rabbit chondrocytes was developed based on a combination of established and our proprietary methods. The rabbit chondrocytes are characterized by immunofluorescence with antibodies specific to collagen II. Each vial contains 0.5x10^6 cells per ml and is delivered frozen.
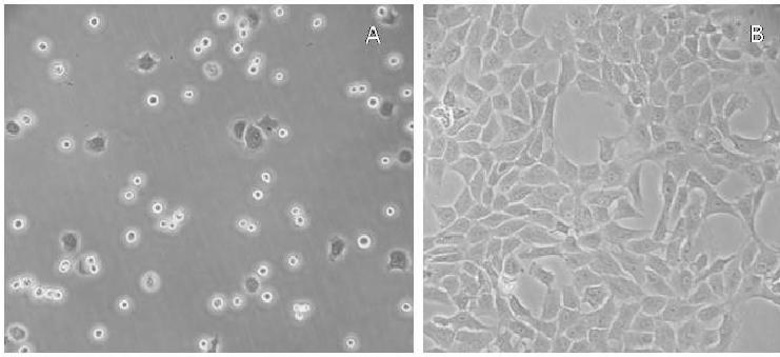
Rabbit chondrocytes are a common primary cell model used to investigate cartilage biology, tissue engineering strategies and joint disease pathomechanisms. The main source of rabbit chondrocytes is New Zealand white rabbits' articular cartilage but extraction can also occur from costal cartilage or vertebral end plate cartilage. After enzymatic digestion, chondrocytes will attach to tissue culture plastic 24-48 h post seeding, as a confluent monolayer of round to polygonal cells that express high levels of type II collagen (COL2A1), aggrecan, and the transcription factor Sox9, which are indicative of the hyaline cartilage phenotype.
In culture, rabbit chondrocytes have an optimal growth rate in DMEM/F 12 media supplemented with 10 % fetal bovine serum or species matched rabbit serum (the latter of which attenuates de-differentiation). Primary chondrocytes maintain their chondrogenic phenotype for 2-3 passages before they undergo gradual transdifferentiation to a fibroblastic morphology, downregulation of COL2A1, and upregulation of type I collagen.
Functionally, rabbit chondrocytes synthesize ECM components, respond to anabolic stimuli like TGF β and IGF 1 and undergo catabolic activation in response to IL 1β or TNF α, which makes them particularly useful for recapitulating inflammation driven cartilage catabolism in vitro. Their responsiveness to mechanical loading, low level laser irradiation, and a variety of pharmacologic compounds is one of the reasons rabbit chondrocytes are often used for scaffold evaluation, 3 D bioprinting and autologous chondrocyte implantation (ACI) studies.
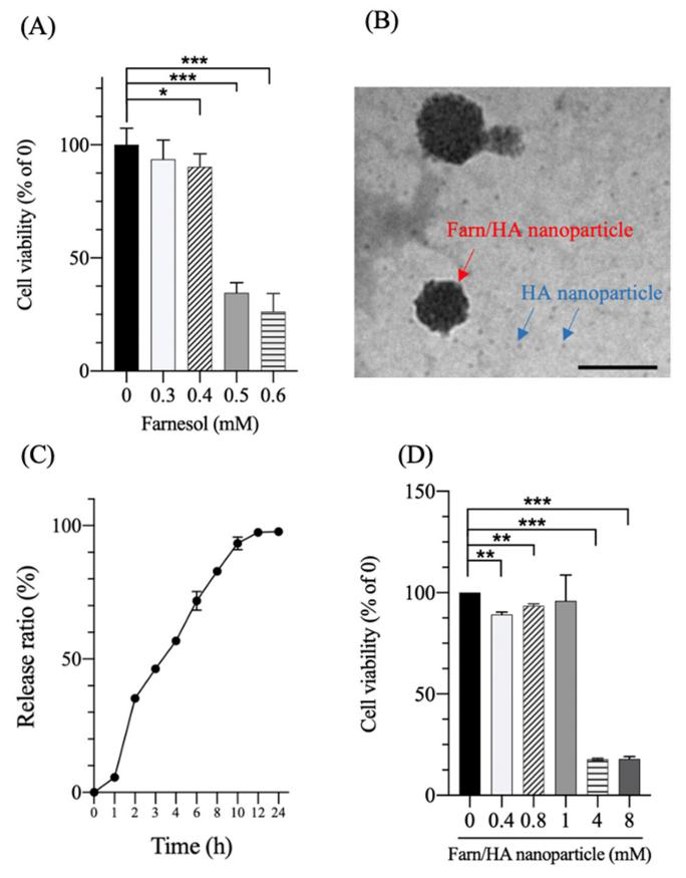
Effect of Farnesol and Farn/HA Nanoparticle on Cell Viability of Rabbit Chondrocytes
Osteoarthritis (OA) is a disease that involves the destruction of articular cartilage. The phenotype and metabolism of chondrocytes are the key of cartilage function. Dedifferentiation, the loss of chondrocyte phenotype and the synthesis of collagen I and X, is a common feature of OA chondrocytes in vitro. Farnesol possesses anti-inflammatory and pro-collagen synthesis properties but its effect on chondrocyte differentiation has not been fully investigated. Wu's group reported an in vitro study to test the potential of farnesol to reverse dedifferentiated chondrocytes' phenotype by measuring the collagen and glycosaminoglycan (GAG) synthesis.
The impact of Farnesol and Farn/HA nanoparticles on cell viability was assessed by MTT assay (Fig. 1A). Farnesol at concentrations of less than 0.4 mM was not significantly detrimental to cell viability after 24 hours. The half inhibitory concentration (IC50) of farnesol was approximately 0.45 mM. The Farn/HA nanoparticles had an average diameter of 58.0 ± 10.4 nm (Fig. 1B), and an encapsulation efficiency (EE) of approximately 90% for farnesol. Approximately 90% of farnesol was released within 10 hours in phosphate-buffered solution (Fig. 1C). However, co-incubation with 4 mM Farn/HA nanoparticles was significantly detrimental to cell viability (Fig. 1D). The IC50 of Farn/HA nanoparticles was approximately 1.9 mM, which is 4.2-fold higher than that of farnesol. These data suggest that HA nanoparticles may abrogate part of the inhibitory effect on cell viability and increase the therapeutic effect by slowing the release of the drug.
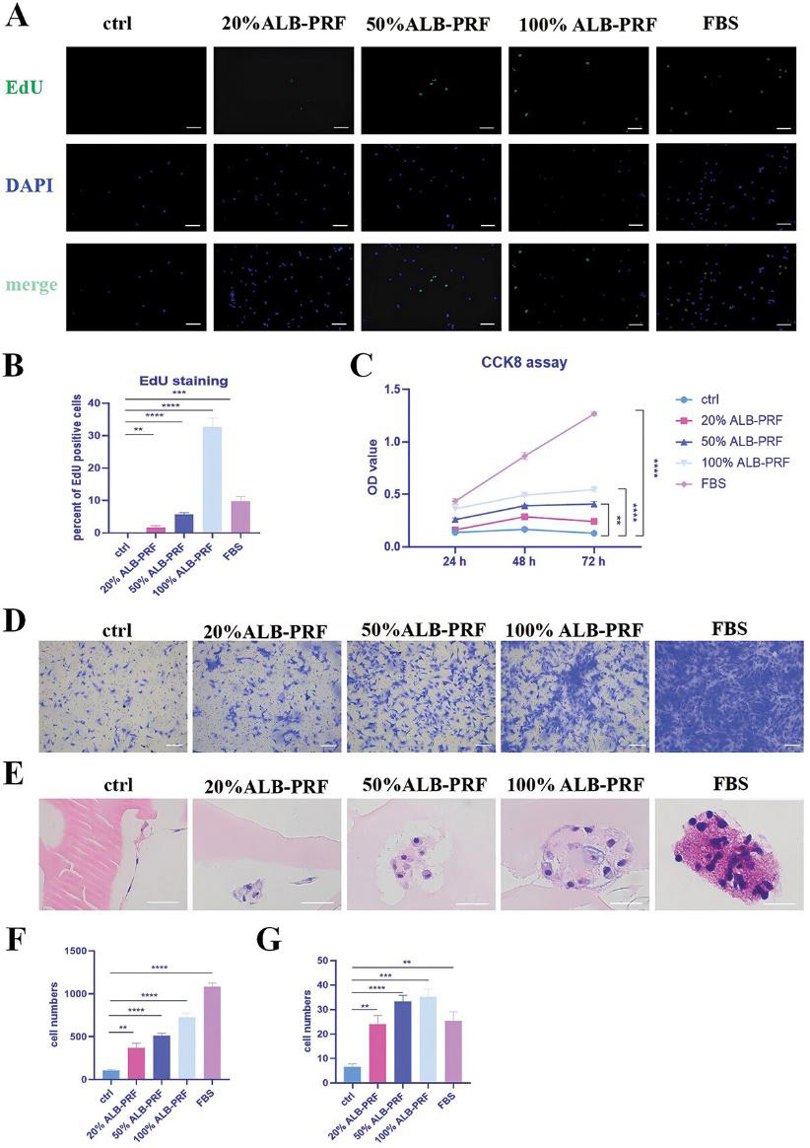
ALB-PRF Conditioned Media Promoting Proliferation, Migration and Adhesion of Rabbit Chondrocytes
Cartilage injury is prevalent in orthopedics, and cartilage tissue engineering offers a therapeutic direction for regeneration. Albumin (ALB)-platelet-rich fibrin (PRF) is theorized as an ideal natural scaffold for cartilage tissue engineering due to its origin from human venous blood. Zeng's team investigated the potential of ALB-PRF as a scaffold material through in vitro and in vivo experiments.
To study the effects of ALB-PRF on chondrocytes, they cultured these cells with different concentrations of ALB-PRF conditioned media. EdU staining showed that 20% ALB-PRF media significantly promoted cell proliferation in a concentration-dependent manner (Fig. 2A, B). The CCK8 assay also confirmed the proliferative effects of ALB-PRF (Fig. 2C). Additionally, transwell experiments showed that ALB-PRF media significantly enhanced chondrocyte migration, also in a concentration-dependent way, though FBS had the strongest effect (Fig. 2D, F). To see if ALB-PRF could serve as a scaffold material for tissue engineering, we tested its ability to support cell adhesion and growth. Cells were directly inoculated onto ALB-PRF and cultured for 10 days. HE staining showed that as the concentration of growth factors increased, cells not only adhered to the ALB-PRF surface but also grew into the inner zone of the gel, degrading the surrounding ALB (Fig. 2E, G).
Ask a Question
Write your own review