Rabbit Aortic Valve Interstitial Cells
Cat.No.: CSC-C5263S
Species: Rabbit
Source: Heart
Cell Type: Interstitial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Rabbit aortic valve interstitial cells from Creative Bioarray are isolated from the rabbit aortic tissue. The method we use to isolate rabbit aortic valve interstitial cells was developed based on a combination of established and our proprietary methods. The rabbit aortic valve interstitial cells are characterized by immunofluorescence with antibodies specific to α-SMA or vimentin. Each vial contains 0.5x10^6 cells per ml and is delivered frozen.
Rabbit Aortic Valve Interstitial Cells (rAVICs) are primary cells derived from the aortic valve tissue of rabbits. These cells constitute the major cell type in the aortic valve and play a crucial role in maintaining valve structure and function. In the native aortic valve, interstitial cells are thought to be the primary mediators of extracellular matrix (ECM) homeostasis and turnover of collagen, elastin, and proteoglycans. They are also highly responsive to biomechanical and biochemical stimuli, making them an important focus of study in understanding aortic valve biology and disease.As primary cells directly isolated from the aortic valve tissue of rabbits, rAVICs provide a physiologically relevant in vitro model system to study normal valve biology as well as aortic valve disease pathophysiology.
Under normal physiological conditions, rabbit aortic valve interstitial cells typically display a quiescent fibroblast-like phenotype. However, in response to inflammatory stimuli, mechanical stress, or pro-calcific conditions, these cells can undergo phenotypic changes towards a myofibroblastic or osteogenic-like phenotype. This phenotypic plasticity makes rAVICs a useful cellular model for studying the cellular and molecular mechanisms underlying aortic valve sclerosis and calcific aortic valve disease (CAVD). Research using rAVICs has helped elucidate signaling pathways involved in aortic valve calcification, fibrosis, oxidative stress, and inflammation, such as TGF-β, BMP, Wnt, and Notch signaling pathways.
ox-LDL Stimulation can Induce Osteoblastic Phenotypic Transformation of Aortic Valve Stromal Cells
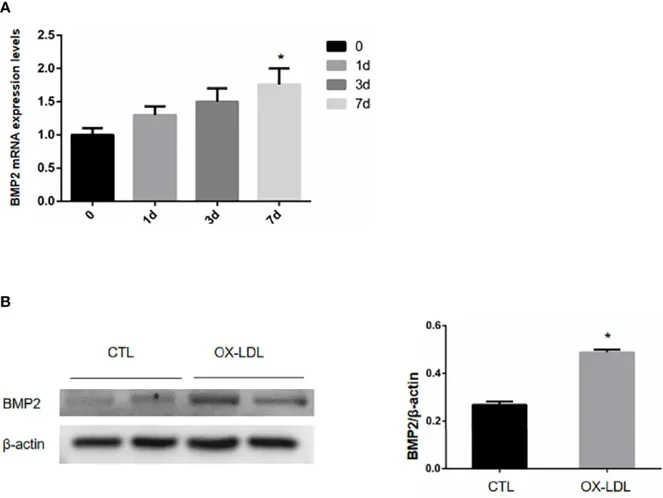
CAVD is a prevalent condition in the elderly. Here, Tao's team isolated and cultured rabbit aortic valvular interstitial cells (VICs) and stimulated them with ox-LDL to induce valvular osteogenic transformation. They assessed the expression of BMP2, PERK, CHOP, and ATF4 after treating the cells with the ER stress inhibitor TUDCA. On day 1, ox-LDL was applied to stimulate aortic valve interstitial cells, which were then collected on days 1, 3, and 7 to measure BMP2 mRNA levels. The BMP2 mRNA content in unstimulated cells served as day 0. Results indicated that BMP2 mRNA increased progressively on days 1, 3, and 7, peaking on day 7 (Fig. 1A). After 7 days of ox-LDL stimulation, VICs were collected and analyzed for BMP2 protein expression via WB. Compared to the CTL group, ox-LDL-stimulated VICs showed elevated BMP2 levels (Fig. 1B), suggesting that ox-LDL induces osteoblastic transformation in these cells.
Ask a Question
Write your own review
