Mouse Dermal Papilla Cells
Cat.No.: CSC-C5393S
Species: Mouse
Source: Hair Follicle
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse dermal papilla cells from Creative Bioarray are isolated from the mouse skin tissue. The method we use to isolate mouse dermal papilla cells was developed based on a combination of established and our proprietary methods. The mouse dermal papilla cells are characterized by immunofluorescence with antibodies specific to fibronectin. Each vial contains 0.5x10^6 cells per ml and is delivered frozen.
Mouse dermal papilla (DP) cells are hair follicle-specific mesenchymal cells that reside at the base of the hair follicle and play an essential role in hair follicle morphogenesis and cyclic regeneration. Dermal papilla cells are derived from the dermal mesenchyme during embryonic development and located within a niche called the dermal papilla, which is surrounded by hair matrix cells. In culture, dermal papilla cells have a fibroblast-like spindle or stellate morphology but have a unique ability to form three-dimensional aggregates. The ability of DP cells to form three-dimensional aggregates is correlated with their hair-inductive ability.
DP cells function as a signaling center by secreting the major regulators of epithelial-mesenchymal interactions, including Wnt/β-catenin, BMPs, and Shh, to control hair follicle stem cell activation and hair shaft formation. They also secrete angiogenic and growth modulatory factors such as VEGF, IGF-1, and HGF to maintain follicle cycling. Dermal papilla cells have been shown to be required for hair follicle regeneration, as they are able to induce de novo hair growth when mixed with epidermal cells and transplanted in drug screening, tissue engineering and other applications.
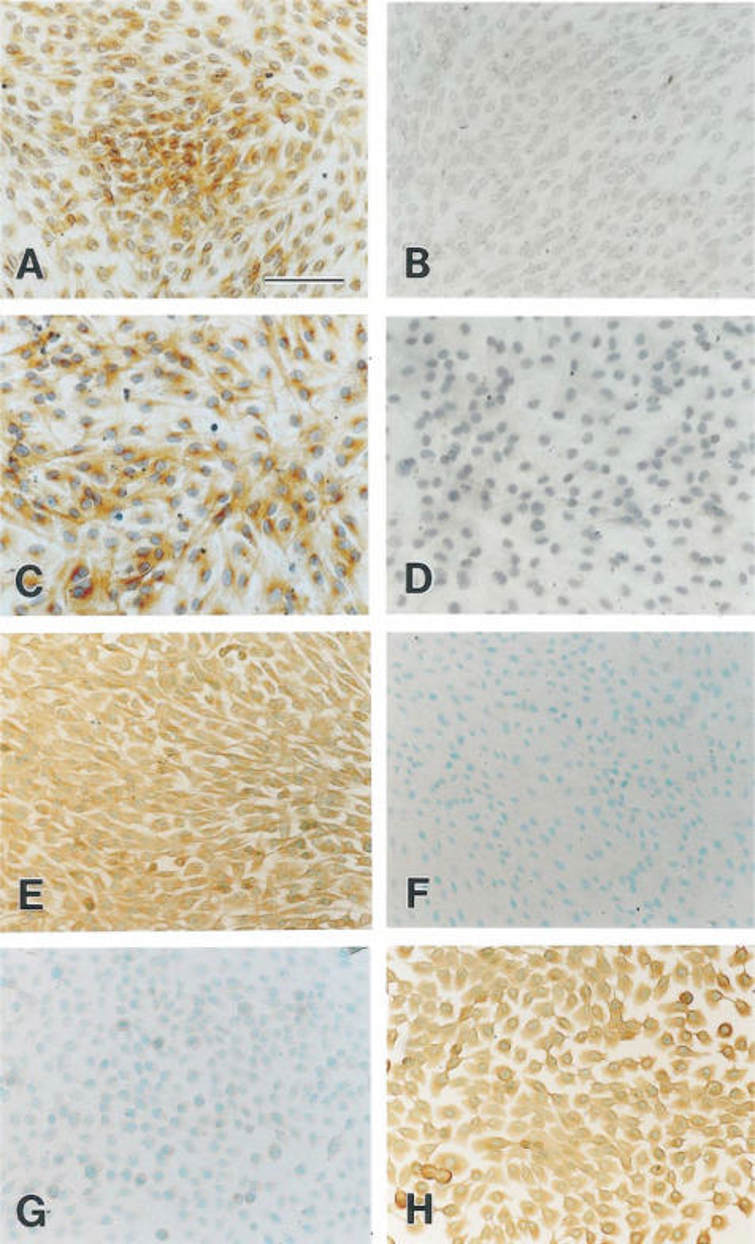
Hordenine Promotes Cell Proliferation and Elevates the Activity of Dermal Papilla Cells
Hair follicles have a growth cycle that can be disrupted, leading to alopecia. Current treatments for alopecia are limited due to inefficiency or side effects. Hordenine, a natural compound with various health benefits, has not been studied for its effects on hair growth. Wang's team aims to investigate whether Hordenine can promote hair growth by enhancing dermal papilla cell (DPC) activity and activating the Wnt/β-catenin signaling pathway.
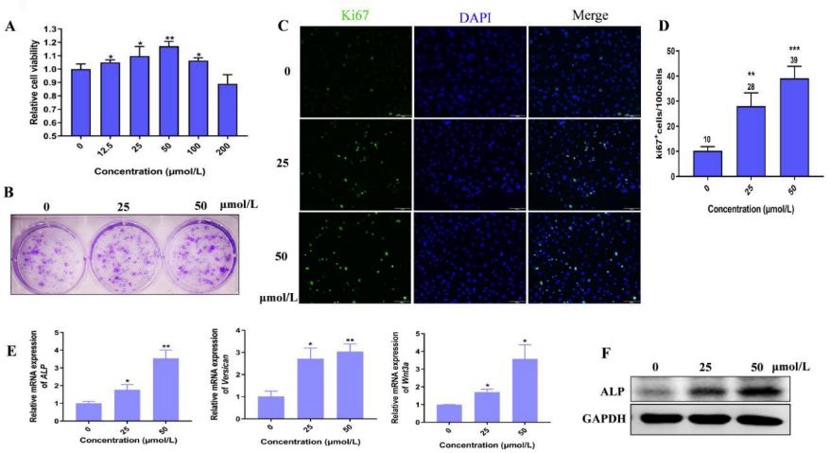
As shown in Fig. 1A, Hordenine at 200 μmol/L was safe for DPCs. Treatment with 12.5-100 μmol/L of Hordenine promoted DPC proliferation, especially 50 μmol/L. The clone formation assay showed that the number of colonies significantly increased by Hordenine (50 μmol/L) compared to controls (Fig. 1B). Immunofluorescence staining analysis indicated more Ki67-positive cells in Hordenine-treated groups than in the control group (Fig. 1C, D). These results demonstrated that Hordenine promoted the proliferation of DPCs. As ALP is an index of DPCs induction potential, they investigated the expression of ALP in DPCs. ALP mRNA and protein levels were upregulated in Hordenine-treated DPCs in a dose-dependent manner (Fig. 1E, F). The mRNA levels of Versican and Wnt3a, which are related to DPC function, were detected. Q-PCR results showed that Hordenine treatment increased the expression of both genes (Fig. 1E). Taken together, these results indicated that Hordenine increased DPC proliferation and DPC induction activity in a dose-dependent manner.
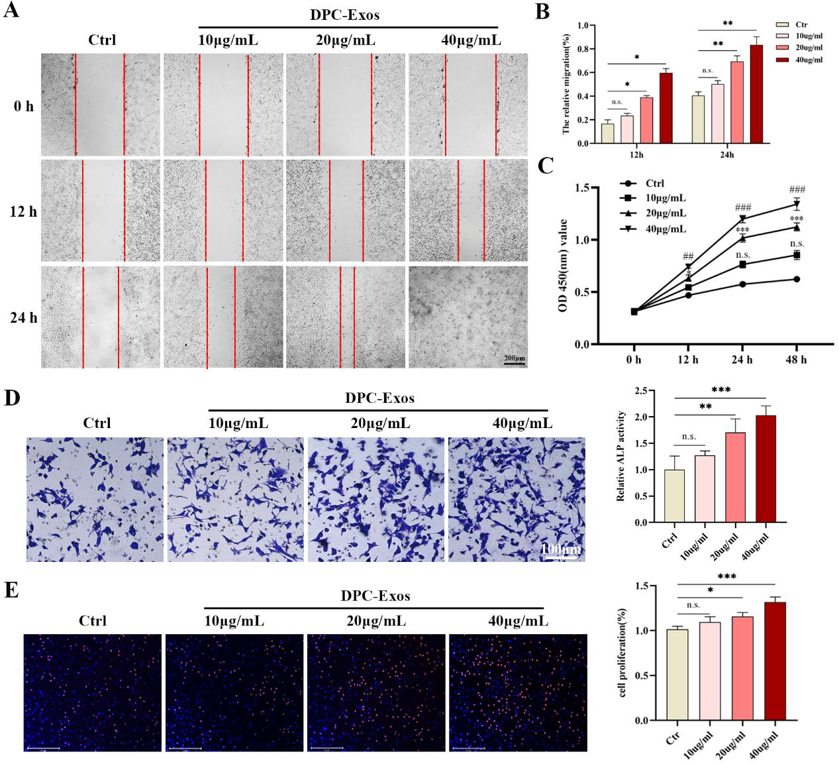
DPC-Exos Promoted the Proliferation and Migration of Fibroblasts in a Concentration-Dependent Manner
Hair follicle (HF) regeneration after skin injury remains a major clinical challenge. Dermal papilla cell-derived exosomes (DPC-Exos) have great potential to induce HF neogenesis. However, the role and mechanism of DPC-Exos in HF regeneration during wound healing are still unclear. In this study, the effect of DPC-Exos on fibroblasts in wound healing was explored for the first time.
Fibroblasts are key cells in dermal skin and crucial for wound healing. DPC-Exos have the ability to promote the proliferation and migration of hair follicle stem cells and hair matrix cells. To verify whether DPC-Exos can also act on fibroblasts, Shang et al. first verified the uptake of DPC-Exos by fibroblasts. The results showed that after co-incubating PKH-26-labeled DPC-Exos with fibroblasts for 24 h, the fibroblasts could be taken up and internalized DPC-Exos. They then treated the fibroblasts with different concentrations of DPC-Exos. As shown in Fig. 2C, the CCK-8 assay showed that DPC-Exos could significantly promote fibroblast proliferation in a concentration-dependent manner, and it was statistically significant at 20 µg/mL and 40 µg/mL, with the most rapid proliferation at 24 h. The same promoting effect was also confirmed by EdU staining (Fig. 2E). The scratch and transwell assays showed that DPC-Exos could improve fibroblast migration in a concentration-dependent manner, and it was statistically significant at 20 µg/mL and 40 µg/mL (Fig. 2A, B, D). These results suggest that DPC-Exos could promote the proliferation and migration of fibroblasts.
Ask a Question
Write your own review