mouse bone marrow macrophages
Cat.No.: CSC-C1940
Species: Mouse
Source: Bone Marrow
Cell Type: Macrophage
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
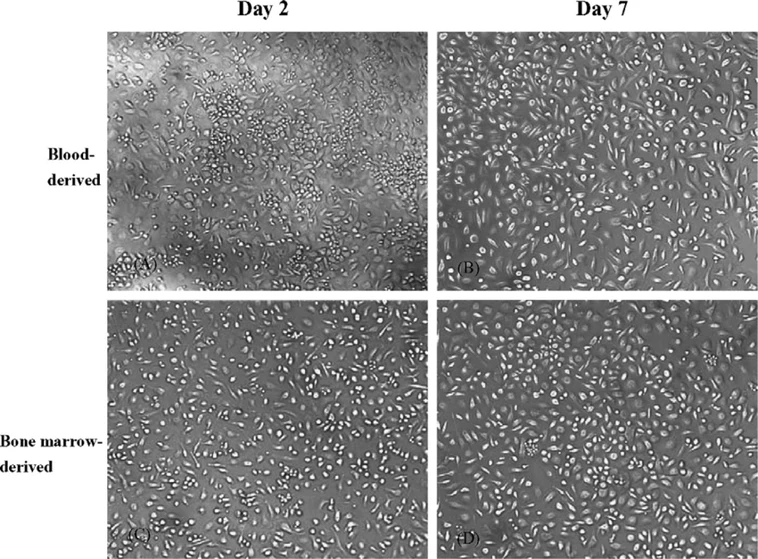
Bone marrow macrophages (BMMs) are primary macrophages isolated from the bone marrow of long bones in mice, such as the femur and tibia. Hematopoietic progenitor cells can be extracted from bone marrow and differentiated ex vivo to produce mature macrophages under continuous stimulation with macrophage colony-stimulating factor (M-CSF). As such, BMMs are a pure and physiologically relevant population that recapitulates the innate immune functions of macrophages in vivo.
BMMs adhere and have a polygonal or elongated shape with extensive cytoplasmic projections typical for professional phagocytes. They express well-known macrophage markers such as F4/80, CD11b, CD68, and MHC-II and are highly phagocytic with robust cytokine responses. BMMs can be polarized toward M1 (pro-inflammatory) or M2 (anti-inflammatory) phenotypes upon stimulation, making them a flexible tool to study macrophage biology and inflammatory signaling pathways. Functionally, BMMs play central roles in antigen processing, microbial clearance, ROS/NO production, and modulation of inflammatory cytokine networks. BMMs have been used to model host-pathogen interactions, innate immune activation, chronic inflammatory diseases, metabolic conditions, and autoimmune disorders due to their physiological relevance and reproducibility.
Role of NLRP3 Inflammasome and IL-1β in Osteoclastogenesis in the Presence and Absence of Lipopolysaccharide (LPS)
The NLRP3 inflammasome is known to promote bone resorption during inflammatory bone diseases like periodontitis by inducing the production of IL-1β. The NLRP3 inflammasome's role in physiological bone remodeling however remains to be determined. Alam et al. investigated the role of the NLRP3 inflammasome in osteoclastogenesis in the presence and absence of LPS.
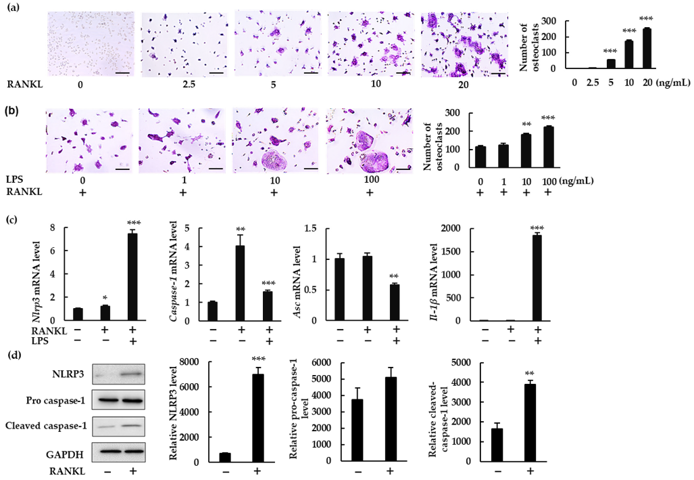
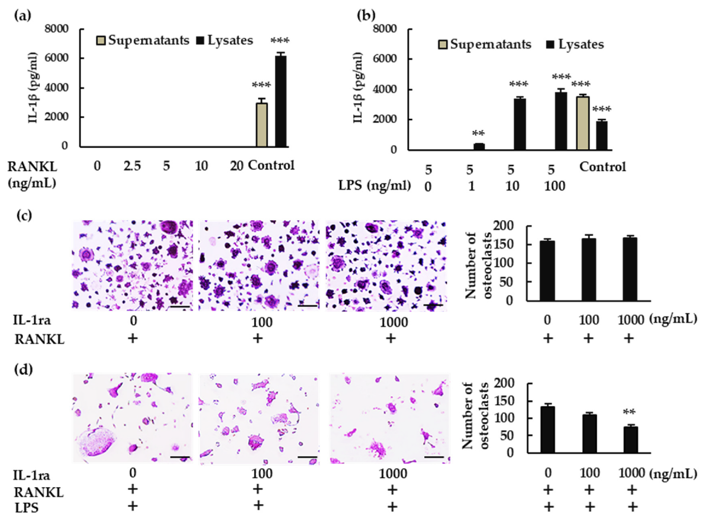
To explore if the NLRP3 inflammasome regulates RANKL-induced osteoclast formation, Alam et al. measured NLRP3 components and IL-1β in mouse bone marrow macrophages (BMMs) treated with RANKL ± LPS. RANKL induced osteoclastogenesis in a dose-dependent manner with M-CSF (Fig. 1a). Treatment with RANKL + LPS increased the number of osteoclasts (Fig. 1b). Treatment with RANKL alone, but not LPS, upregulated Nlrp3 and Caspase-1 mRNA expression but not Asc or Il-1β (Fig. 1c) and increased NLRP3 and cleaved caspase-1 protein (Fig. 1d). With RANKL + LPS, all components (Nlrp3, Caspase-1, and Il-1β) were upregulated while Asc was downregulated. From these data, it can be concluded that RANKL is able to upregulate Nlrp3 and Caspase-1 but not Il-1β without LPS. Next, they analyzed whether IL-1β plays a role in BMMs. RANKL alone did not induce IL-1β in the supernatant or lysate (Fig. 2a). In the presence of LPS, IL-1β was detected in the lysate but not the supernatant (Fig. 2b). Alam et al. then tested the effect of recombinant IL-1 receptor antagonist (rIL-1ra) and determined that it did not affect osteoclast formation without LPS (Fig. 2c) while inhibiting it with LPS (Fig. 2d). These data suggests that IL-1β is required for osteoclastogenesis in the presence of LPS but not in its absence.
Ask a Question
Write your own review