Mouse Hepatocytes
Cat.No.: CSC-C2125
Species: Mouse
Source: Liver
Cell Type: Hepatocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse hepatocytes are mouse primary cells derived directly from the parenchyma of the liver rather than from immortalized cell lines. Mouse hepatocytes are epithelial cells that functionally are the liver's most abundant cells, making up around 80% of all liver cells. In the in vivo setting, hepatocytes are organized into plates of polarized cells with basolateral membranes that interface with the sinusoids for nutrient exchange, and apical membranes that are contiguous to other hepatocytes' apical membranes, which form bile canaliculi for biliary excretion.
Mouse hepatocytes in vitro, once isolated by collagenase perfusion in two steps and cultured, typically form a confluent monolayer with a polygonal shape. They are non-dividing in conventional culture and become progressively dedifferentiated over the course of one to two weeks, during which time they lose various functional attributes. Functionally, they are active in the homeostasis of glucose and lipids, synthesis of plasma proteins like albumin, and biotransformation of nutrients, drugs, and xenobiotics. These cells are particularly enriched with cytochrome P450 enzymes and are therefore a model of choice for the metabolism of xenobiotics, as well as for studies of drug toxicity and pharmacokinetics. Consequently, primary mouse hepatocytes are used as an ex vivo model extensively in biomedical research. Their use ranges from drug discovery and toxicology to understanding mechanisms of liver disease including steatosis, hepatitis, metabolism, and the use of genetically engineered mice to study basic pathological processes.
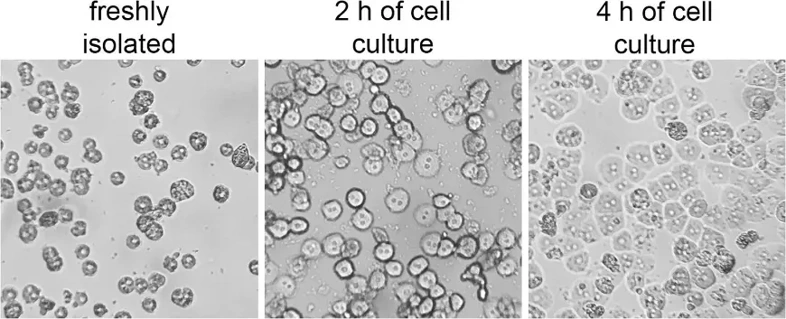
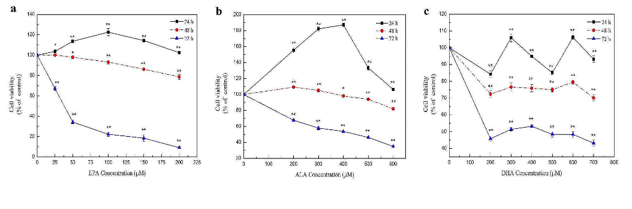
Effects of Different n-3 PUFAs on Cell Proliferation of Mouse Hepatocytes
Omega-3 polyunsaturated fatty acids (n-3 PUFAs) have antioxidant activity and are essential for growth and development. Wang's team investigated the combined effects of ALA, EPA, and DHA on the viability and antioxidant capacity of mouse hepatocytes to obtain the best combination for improving antioxidant capacity.
To assess the impact of EPA, ALA, and DHA on cell proliferation, in vitro experiments were conducted using NCTC 1469 mouse hepatocytes. Results showed significant effects of n-3 PUFAs on cell viability. EPA treatment for 24 h led to an initial increase then decrease in cell viability, peaking at 24 h and dropping to about 80% at 48 h. After 72 h, viability sharply decreased to about 10%, with most cells dying at 50 µM EPA (Fig. 1a). ALA treatment for 24 h significantly increased cell viability at 200-600 µM, peaking at 400 µM, higher than EPA and DHA treatments. After 48 h, ALA had minimal effect, and 600 µM ALA reduced viability to less than half after 72 h (Fig. 1b). DHA treatment led to fluctuating cell viability after 24 h, significantly decreasing to about 80% and 40% after 24 h and 48 h, respectively (Fig. 1c). For subsequent experiments focusing on antioxidant capacity, concentrations and treatment times with minor viability decreases were selected: 0-200 µM EPA, 0-600 µM ALA, and 0-700 µM DHA for 24 h.
Ask a Question
Write your own review