C57BL/6 Mouse Primary Esophageal Epithelial Cells
Cat.No.: CSC-C4268X
Species: Mouse
Source: Esophagus
Cell Type: Epithelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse Primary Esophageal Epithelial Cells can be used in assays of cell to cell adhesion and migration. Standard biochemical procedures performed with epithelial cell cultures include RT-PCR, Western blotting, immunoprecipitation, immunofluorescent staining or immunofluorescent flow cytometry or generating cell derivatives for desired research applications.
Mouse Primary Esophageal Epithelial Cells (MPEECs) are isolated directly from the murine esophagus, representing a non-transformed, physiologically relevant in vitro model that closely mirrors the in vivo state of the esophageal lining. Their key characteristics include being finite-lived in culture, maintaining their original genotype and phenotype, and expressing specific markers of esophageal epithelial differentiation, such as cytokeratins. MPEECs are indispensable for exploring the cellular and molecular mechanisms underlying esophageal pathologies, including carcinogenesis, the response to injury (such as acid reflux), and Barrett's esophagus metaplasia. Their main advantage lies in providing a more authentic platform compared to immortalized cell lines, as they avoid the genetic alterations associated with continuous passage, thereby yielding data with higher translational relevance for drug discovery, toxicology studies, and understanding disease mechanisms in a controlled experimental setting.
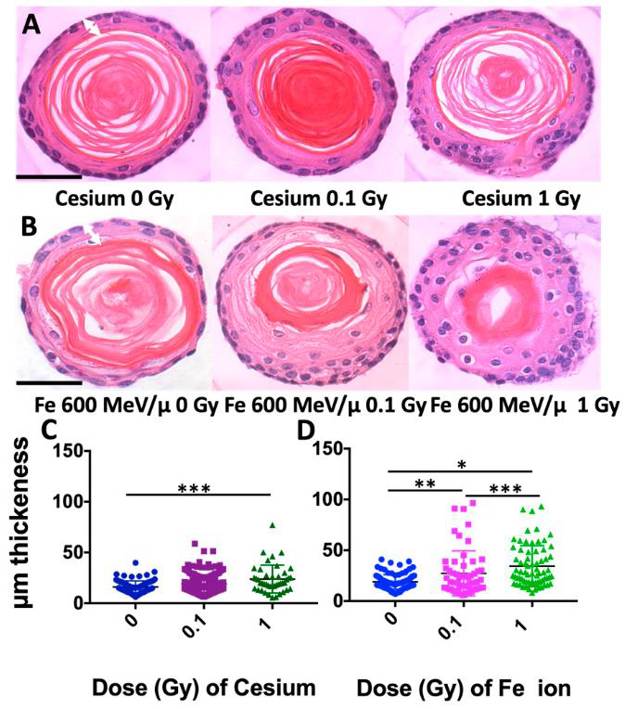
Radiation Quality and Dose Effects in Mouse Esophageal Organoids
Esophageal squamous cell carcinoma (ESCC) is a deadly consequence of radiation exposure to the esophagus. ESCC arises from esophageal epithelial cells that undergo malignant transformation and features a perturbed squamous cell differentiation program. Understanding the dose- and radiation quality-dependence of the esophageal epithelium response to radiation may provide insights into the ability of radiation to promote ESCC.
Carswell, Latisha, et al. used a murine three-dimensional (3D) organoid model that recapitulates the morphology and functions of the stratified squamous epithelium of the esophagus to study persistent dose- and radiation quality-dependent changes. Interestingly, although high-linear energy transfer (LET) Fe ion exposure induced a more intense and persistent alteration of squamous differentiation and 53BP1 DNA damage foci levels as compared to Cs, the MAPK/SAPK stress pathway signaling showed similar altered levels for most phospho-proteins with both radiation qualities. In addition, the lower dose of high-LET exposure also revealed nearly the same degree of morphological changes, even though only ~36% of the cells were predicted to be hit at the lower 0.1 Gy dose, suggesting that a bystander effect may be induced. Although p38 and ERK/MAPK revealed the highest levels following high-LET exposure, the findings reveal that even a low dose (0.1 Gy) of both radiation qualities can elicit a persistent stress signaling response that may critically impact the differentiation gradient of the esophageal epithelium, providing novel insights into the pathogenesis of radiation-induced esophageal injury and early-stage esophageal carcinogenesis.

Ask a Question
Write your own review