C57BL/6 Mouse Primary Aortic Fibroblasts
Cat.No.: CSC-C4275X
Species: Mouse
Source: Aorta
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse Primary Aortic Fibroblasts are negative for bacteria, yeast, fungi, and mycoplasma. Cells are tested for expression of marker using the antibody of anti-FSP1/S100A4 by immunofluorescence staining. Cells can be expanded for 2-4 passages at a split ratio of 1:2 under the cell culture conditions specified by Creative Bioarray. Repeated freezing and thawing of cells is not recommended.Standard biochemical procedures performed with cell cultures include the assay of cell to cell interaction, RT-PCR, Western blotting, immunoprecipitation, immunofluorescent staining, flow cytometry or generating cell derivatives for desired research applications.
C57BL/6 Mouse Primary Aortic Fibroblasts are primary stromal cells derived from murine aortic tissue. They can be used as a physiologically relevant in vitro model system to study vascular connective tissue biology. Because aortic fibroblasts comprise the majority of non-endothelial cell populations in the aortic wall, they are essential for extracellular matrix (ECM) homeostasis and provide structural support to the vessel.
Mouse Primary Aortic Fibroblasts maintain a spindle-shaped fibroblast-like morphology and demonstrate healthy adherence and proliferation in vitro. Like most fibroblasts they express cell markers including vimentin, collagen I, collagen III and fibronectin. Additionally, these cells can undergo activation into myofibroblasts when stimulated with profibrotic factors which can be accompanied by expression of α-smooth muscle actin (α-SMA) and production of ECM. Overall, Mouse Primary Aortic Fibroblasts serve as a great model system to study the mechanisms underlying vascular fibrosis and remodeling.
Mouse Primary Aortic Fibroblasts can be used to study a variety of biological processes including aortic development, vascular injury and repair, vascular inflammation, atherosclerosis, aneurysm formation, and hypertension. They can also be used to assess the effect of cytokines, growth factors, and compounds on fibroblast activation and extracellular matrix remodeling. Due to their primary nature, these cells offer significant biological relevance to cardiovascular studies and translational studies into vascular disease.
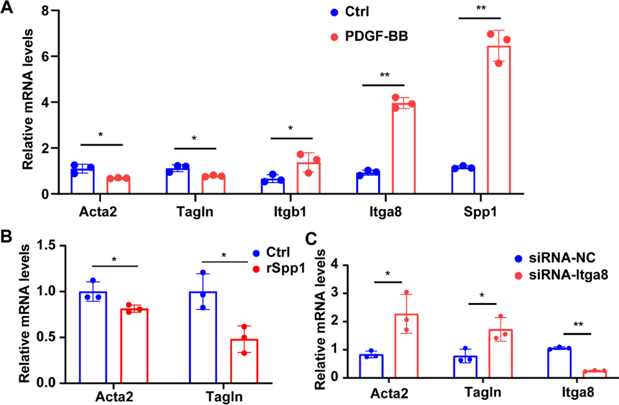
Spp1 Mediates Decline of SMC Contractile Phenotype via Itga8/Itgb1
Fbn1 mutation leads to MFS and TAA, but the mechanisms disrupting ECM homeostasis are unknown. Here, Zhou's team used transcriptome sequencing and single-cell sequencing to identify Spp1 as an ECM-related gene increased in Fbn1C1041G/+ mice. Immunostaining confirmed elevated Spp1 in adventitial fibroblasts. They then explored Spp1's role in mouse aortic fibroblast and smooth muscle cell (SMC) communication through Itga8/Itgb1 and its effects on contractile and collagen gene expression.
The decline of the SMC contractile phenotype is linked to thoracic aortic aneurysm formation. They first stimulated mouse SMCs with PDGF-BB, which induces SMC phenotype switching, and observed increased expression of Itga8, Itgb1, and Spp1 (Fig. 1A), indicating enhanced Spp1-receptor signaling during this transition. Next, we treated mouse vascular fibroblasts and SMCs with recombinant Spp1 protein, finding increased Col1a1 and Col3a1 expression in fibroblasts and decreased Acta2 and Tagln expression in SMCs (Fig. 1B). Since Itga8/Itgb1 mediate Spp1's major effects in MFS (Marfan Syndrome) aortic aneurysms, we knocked down Itga8 in SMCs using siRNA. This abolished the Spp1-induced decrease in Acta2 and Tagln expression (Fig. 1C). Thus, Spp1 reduces the SMC contractile phenotype via Itga8/Itgb1. However, it's unclear if these Spp1 changes in MFS thoracic aortic aneurysms apply to other TAA (Thoracic Aortic Aneurysm) models.
Ask a Question
Write your own review