C57BL/6 Mouse Gingival Epithelial Cells
Cat.No.: CSC-C9083J
Species: Mouse
Source: Gingiva; Periodontium
Cell Type: Epithelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
C57BL/6 Mouse Gingival Epithelial Cells (GECs) are primary cells that have been isolated from the gingival mucosa of C57BL/6 mice. C57BL/6 mice are a common inbred strain used in immunological and periodontal studies. The gingiva is composed of stratified epithelium which can be divided into the keratinized oral epithelium, the semi-permeable sulcular epithelium, and the non-keratinized junctional epithelium (JE). The JE forms a tight seal with the tooth surface and is responsible for regulating the entry of bacteria into the gingival crevice. GECs in vitro show a typical epithelial cobblestone morphology and express epithelial markers including cytokeratins and E-cadherin. They require specialized media with added growth factors to proliferate in vitro and maintain their physiological properties. In their physiological context, GECs serve as a barrier to oral pathogens and are involved in innate immune responses, such as the production of antimicrobial peptides (e.g. β-defensins) and the secretion of cytokines (e.g. IL-6, IL-8). Dysregulation of GECs is thought to contribute to the pathogenesis of periodontitis, with increased inflammatory signaling and tissue destruction observed.
GECs are commonly used in research to study periodontal disease, host-microbe interactions (e.g. in the context of Porphyromonas gingivalis infection), and screening of potential therapeutic agents. They are also used in tissue engineering approaches for gingival regeneration studies. Their genetic background (C57BL/6) allows for reproducibility between experiments and translational relevance for studying epithelial immunity and developing new treatments for periodontal diseases.
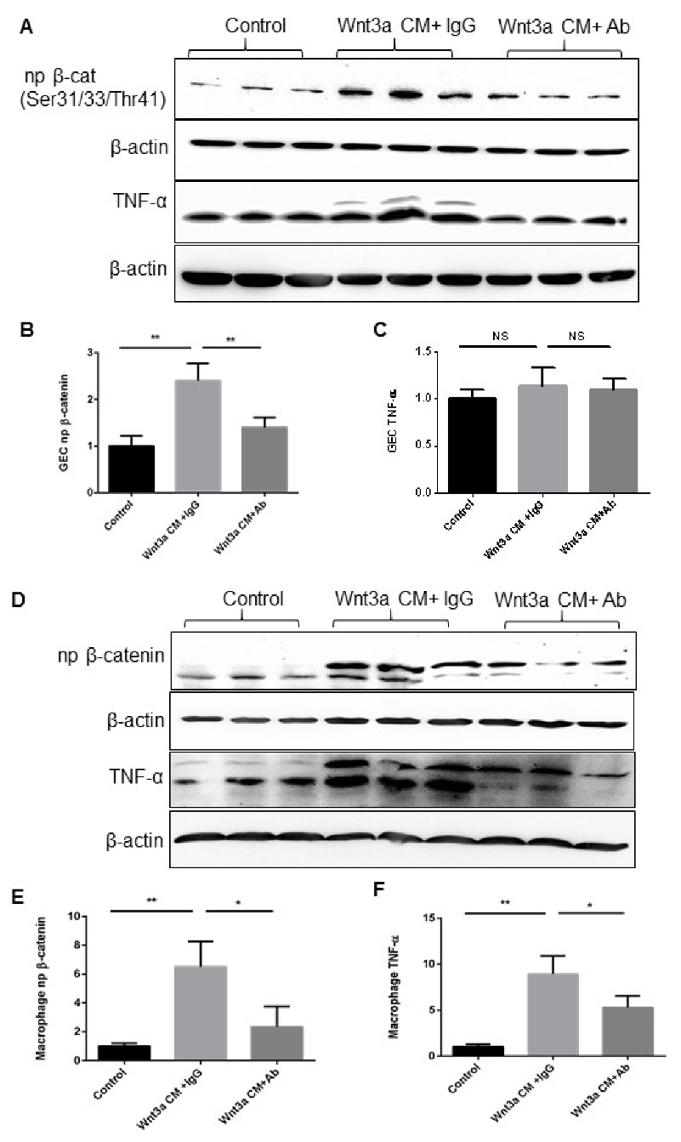
Activation of Canonical Wnt Signaling Induced TNF-α Expression in the Macrophages but Not in GEC In Vitro
Wnt signaling is involved in homeostasis and inflammation, but its role in periodontitis macrophages is unknown. Here, Chen's team induced periodontitis in mice with P.g-associated ligature and examined TNF-α, β-catenin, and F4/80 expression. They assessed Wnt signaling's effect on TNF-α in Raw 264.7 macrophages and compared it with gingival epithelial cells (GECs).
Wnt signaling is vital for macrophage recruitment. They found similar expression patterns of F4/80, activated β-catenin, and TNF-α in gingiva during periodontitis, suggesting that Wnt pathway activation is linked to macrophage recruitment and activation. They treated GEC and Raw 264.7 cells with Wnt3a-conditioned medium, observing nuclear β-catenin accumulation, confirming Wnt pathway activation (Fig. 1A-E). TNF-α significantly increased in Raw 264.7 cells but not in GEC (Fig. 1C, F), indicating that Wnt pathway activation mainly affects macrophages. Blocking Wnt3a with an antibody reduced β-catenin accumulation and significantly decreased TNF-α in Raw 264.7 cells, with no significant change in GEC (Fig. 1A-D, F). This shows that Wnt pathway activation directly promotes TNF-α production in macrophages. Overall, Wnt pathway activation is associated with macrophage recruitment and activation in gingiva during periodontitis, contributing to inflammation in periodontal diseases.
Ask a Question
Write your own review