C57BL/6 Mouse Aortic Endothelial Cells
Cat.No.: CSC-C1861
Species: Mouse
Source: Aorta
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
C57BL/6 Mouse Aortic Endothelial Cells represent primary endothelial cells that scientists derive from the aorta of C57BL/6 mice. These cells serve as an in vitro model system for vascular biology research and understanding the mechanisms of cardiovascular diseases. Endothelial cells (ECs) line the interior surface of blood vessels and are involved in various physiological and pathological processes. Characteristics like supporting vasomotion, vascular permeability, inflammation, and thrombosis make them ideal models for studying endothelial function and dysfunction.
C57BL/6 mouse aortic endothelial cells grown in vitro develop the characteristic cobblestone morphology and establish contact-inhibited monolayer formations. These cells express endothelial cell markers such as CD31/PECAM-1, VE-cadherin, von Willebrand factor (vWF), and endothelial nitric oxide synthase (eNOS). Mouse aortic endothelial cells respond to physical and chemical stimuli such as shear stress, cytokines, and reactive oxygen species. Additionally, they play an active role in angiogenic signaling pathways and vascular remodeling processes. Isolated from a common inbred mouse strain (C57BL/6), they have good genetic uniformity and are compatible with many mouse disease models. C57BL/6 mouse aortic endothelial cells are widely used to study atherosclerosis, hypertension, endothelial inflammation, diabetes, and drug-induced toxicity.
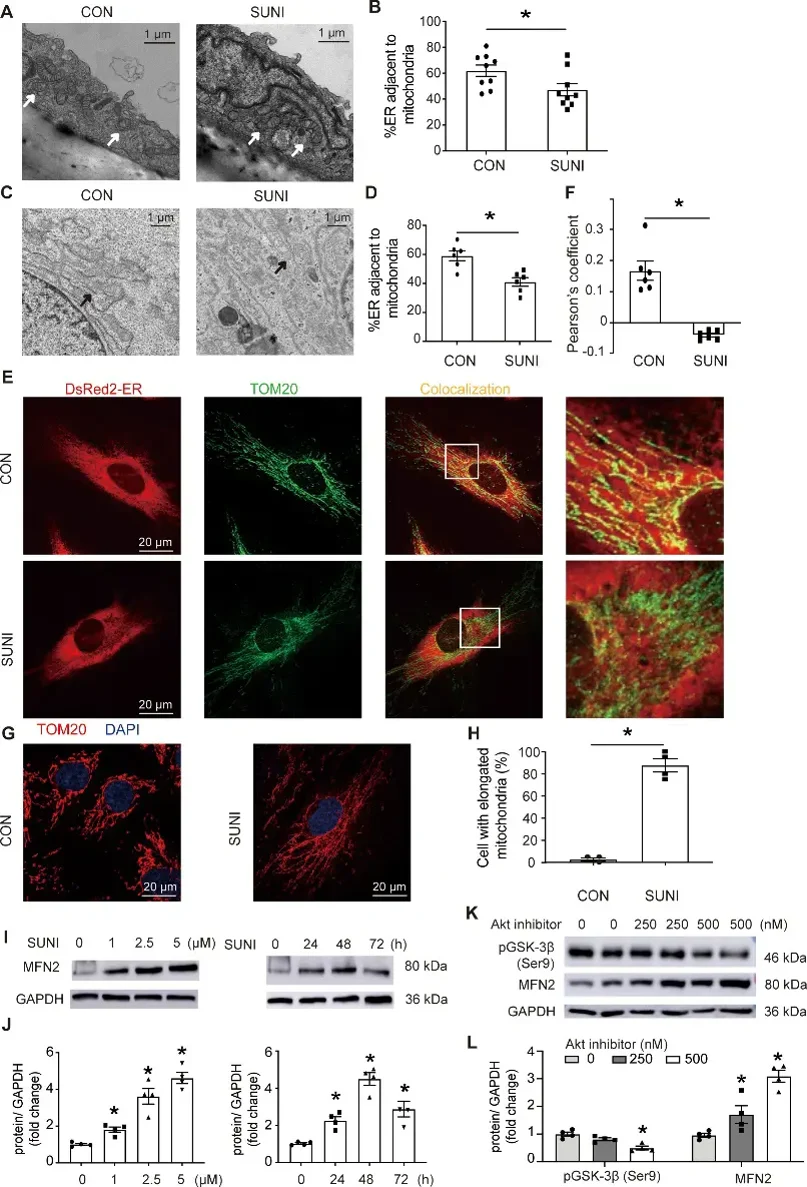
Sunitinib Leads to Decrease in Mams in Mouse Aortic Endothelial Cells and HUVECs
TKIs like sunitinib cause hypertension, but the mechanisms are unknown. Here, Qu's team performed in vitro and in vivo experiments to assess ROS, NO, endothelium-dependent vasorelaxation, blood pressure, and mitochondrial function in HUVECs and C57BL/6 mouse aortic endothelial cells under sunitinib treatment. Results showed that sunitinib increased mitochondrial ROS, decreased OCR, ATP production, and [Ca2+]M levels, and impaired eNOS and NO signaling.
Purifying MAMs (Mitochondria-Associated Membranes) has shown a physical link between the ER (Endoplasmic Reticulum) and mitochondria, with gaps of 6-15 nm. MAMs are vital for mitochondrial function, so we tested if sunitinib affects them. TEM (Transmission Electron Microscopy) showed sunitinib reduced MAMs in mouse aortic endothelial cells and HUVECs (Human Umbilical Vein Endothelial Cells) (Fig. 1A-D). Confocal images also showed less ER-mitochondria overlap and mitochondrial elongation in sunitinib-treated HUVECs (Fig. 1E-H).
Ask a Question
Write your own review