- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse Cells are negative for bacteria, yeast, fungi, and mycoplasma. Repeated freezing and thawing of cells is not recommended.Standard biochemical procedures performed with cell cultures include RT-PCR, Western blotting, immunoprecipitation, immunofluorescent staining, flow cytometry or generating cell derivatives for desired research applications.
Never can primary cells be kept at -20 °C.
BALB/c Mouse Primary Heart Cells is a cardiac cell population derived from the hearts of BALB/c mice. BALB/c mouse primary heart cells can be used as an in vitro model system that closely resembles the physiology of the heart. Mouse primary heart cells are usually composed of cardiomyocytes. However, depending on how they are isolated, the cell pellet can also contain cardiac fibroblasts, cardiac endothelial cells and smooth muscle cells.
Characteristics of mouse primary heart cells include expression of cardiac markers like cardiac troponin T (cTnT), α-actinin and connexin-43. Spontaneous contraction of cardiomyocytes can also be observed when cells are seeded under optimal conditions. Mouse primary heart cells are useful for studying cardiac physiology such as cardiac development, excitation-contraction coupling, and calcium handling. Because mouse primary heart cells are primary cells from an inbred mouse strain, they have a high degree of relevance and are used in many applications. They can be used to study cardiac toxicity, ischemia-reperfusion, cardiac fibrosis, inflammation and testing drug candidates for cardioprotective or cardiotoxic effects.
Heart Spheroids from Primary Cells for Studying Cardiac Development
Organoids generated from primary cells have been reported for multiple organs including liver, intestine, and colon. These organoids are useful for disease modeling and drug screening studies. However, organoids from primary heart cells have not been reported.
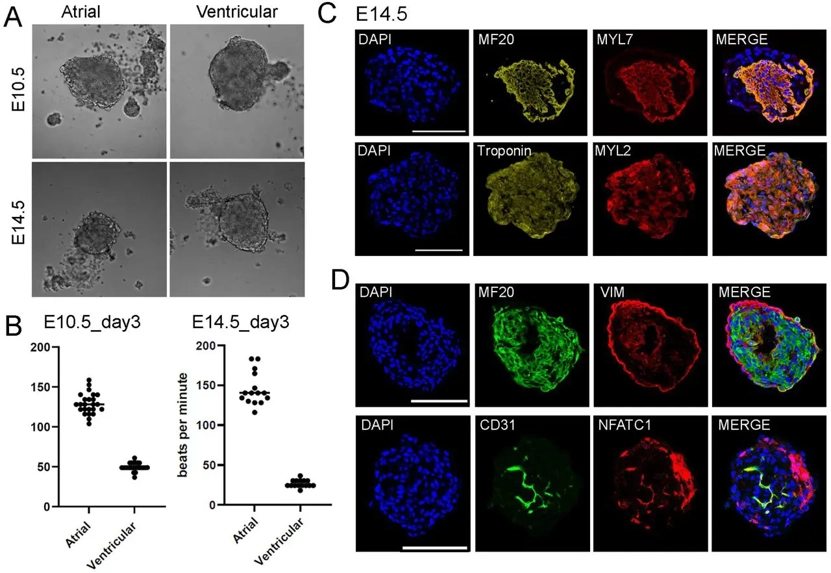
To generate heart spheroids, Chang's team used mouse primary heart cells from early embryonic stages and aggregated them in ultra-low attached 96-well plates. They tested out the spheroids on days 4, 7, and 12, and discovered spheroids with 10k cells in IMDM-based media grew round morphologies with no dark spots (no cell death) and could be used for further testing. Under this optimized condition, they generated atrial and ventricular cardiac cell spheroids at E10.5 and E14.5. They found that atrial spheroids spontaneously beat around 140bpm while ventricular cardiomyocyte spheroids beat under 50 bpm (Fig 1A, B). They performed immunofluorescence (IF) analysis to examine cardiac lineage gene markers. In atrial spheroids, they detected the general cardiomyocyte (CM) marker MF20 and the atrial-specific CM marker Myl7. In ventricular spheroids, we detected the general CM marker Troponin and the ventricular-specific CM marker Myl2 (Fig 1C). They confirmed atrial CM identity using another marker, Nr2f2 (Fig S2A). We also analyzed the distribution of different cell lineages. MF20+ CMs were evenly distributed, while Vim+ fibroblasts were mainly on the edges. Cd31+ endothelial cells, identified by Nfatc1, were mostly located in the middle of the spheroids (Fig 1D).
Ask a Question
Write your own review