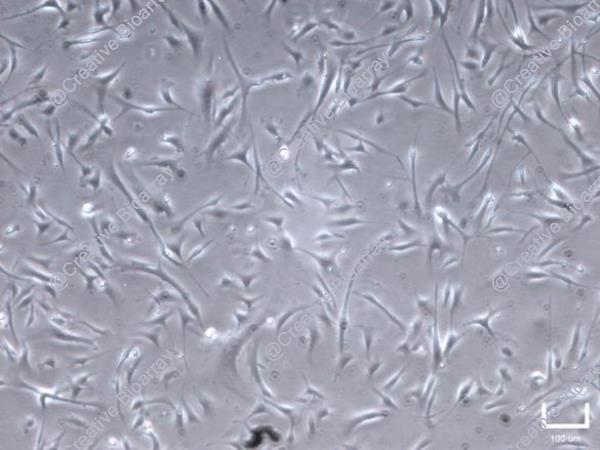
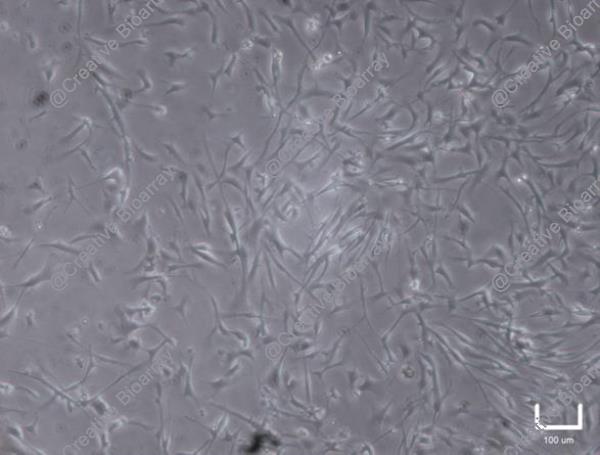
Immortalized Human Primary Trabecular Meshwork Cells-SV40
Cat.No.: CSC-I9168L
Species: Homo sapiens
Source: Juxtacanalicular and corneoscleral regions of human eye
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
- Documents
Note: Never can cells be kept at -20°C.
Human trabecular meshwork cells (TMCs) play a crucial role in aqueous humor outflow, a pathway influenced by intraocular pressure. Injury or death of TMCs, therefore, is associated with the pathogenesis of open-angle glaucoma. TMCs also express receptors for neurotransmitters and neuropeptides. They respond to extremely low concentrations of various vasoactive peptides and growth factors, enabling these cells to regulate permeability of trabecular meshwork at multiple levels. TMC cultures provide an invaluable tool for studying the functional control of the trabecular meshwork.
Immortalized Human Primary Trabecular Meshwork Cells are derived from the normal juxtacanalicular and corneoscleral tissue regions of the human eye and immortalized with SV40. These cells retain key characteristics of primary cells, including expression of specific markers like myocilin and the ability to respond to glucocorticoids and oxidative stress, which are central to the pathophysiology of glaucoma. Their primary application is in modeling the outflow pathway of the eye to study the pathogenesis of glaucoma, particularly primary open-angle glaucoma (POAG). The major advantage of using immortalized human TMCs over primary cells is their consistent availability, genetic stability, and reproducibility, which overcomes the limitations of finite primary cell cultures, donor-to-donor variability, and limited expansion capacity, thereby accelerating the pace of ophthalmic discovery.
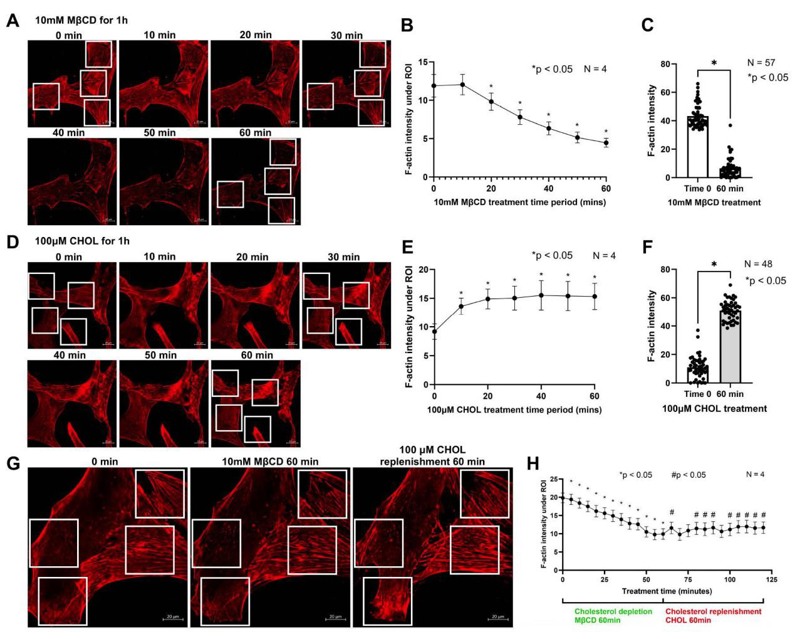
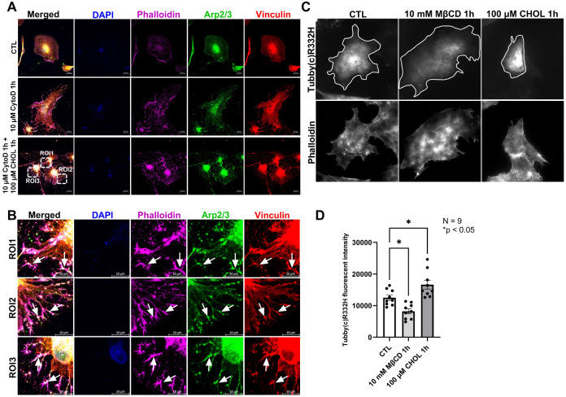
The Remodeling of Actin Cytoskeleton Depends on Cholesterol Levels in HTM Cells
The trabecular meshwork (TM) tissue plays a crucial role in maintaining intraocular pressure (IOP) homeostasis. Increased TM contractility and stiffness are directly correlated with elevated IOP. Although cholesterol is known to be a determinant of glaucoma occurrence and elevated IOP, the underlying mechanisms remain elusive. In this study, we used human TM (HTM) cells to unravel the effects of cholesterol on TM stiffness. We achieved this by performing acute cholesterol depletion with Methyl-β-cyclodextrin (MβCD) and cholesterol enrichment/replenishment with MβCD cholesterol complex (CHOL). Interestingly, cholesterol depletion triggered notable actin depolymerization and decreased focal adhesion formation, while enrichment/replenishment promoted actin polymerization, requiring the presence of actin monomers.
Using a specific reporter of phosphatidylinositol 4,5-bisphosphate (PIP2), we demonstrated that cholesterol depletion decreases PIP2 levels on the cell membrane, whereas enrichment increases them. Given the critical role of PIP2 in actin remodeling and focal adhesion formation, we postulate that cholesterol regulates actin dynamics by modulating PIP2 levels on the membrane. Overall, our systematic exploration of cholesterol modulation on TM stiffness highlights the critical importance of maintaining appropriate membrane and cellular cholesterol levels for achieving IOP homeostasis.
These are human trabecular meshwork cells that have been genetically modified to proliferate using SV40 large T antigen. They provide a robust in vitro model for studying the biology of the trabecular meshwork and its role in ocular diseases.
Immortalized Human Primary Trabecular Meshwork Cells-SV40 (Cat No.: CSC-I9168L) are ideal for studying eye disorders such as glaucoma, intraocular pressure regulation, aqueous humor outflow, and the trabecular meshwork's response to pharmaceutical agents.
Immortalized Human Primary Trabecular Meshwork Cells-SV40 (Cat No.: CSC-I9168L) offer a stable and consistent alternative to primary cells, which have a limited lifespan and variability. This allows for reproducible long-term studies and consistent data generation.
Yes, their stable phenotype and extended lifespan make them suitable for drug screening and testing potential glaucoma treatments, particularly those targeting trabecular meshwork function.
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells