
Immortalized Human Mammary Fibroblasts (HMF3A)
Cat.No.: CSC-I9260L
Species: Homo sapiens
Source: Mammary Tissue
Morphology: Spindle
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT013 HT® Lenti-hTERT Immortalization Kit
CIK-HT003 HT® Lenti-SV40T Immortalization Kit
2) Flow cytometry confirmed expression of T antigen.
Immortalized Human Mammary Fibroblasts (HMF3A) are a conditionally immortal human fibroblast cell line from normal mammary (breast) tissue. They were generated by dual transduction with a temperature sensitive mutant of SV40 large T antigen (U19tsA58), and the catalytic subunit of human telomerase (hTERT). Morphologically the HMF3A are adherent, spindle-shaped fibroblasts. Population doubling time is approximately 57-67 hours at optimal conditions. One of the most potent uses of this cell line is in senescence analysis: by switching temperatures, researchers can control the activation state of the T antigen and thereby induce a senescent program. Indeed, transcriptomic microarrays coupled to RNA interference and in-silico promoter modeling have found bona fide senescence regulators such as DUSP1, NR4A3 and novel splice variants, as well as transcription factor networks of NF-κB and C/EBP.
The cell line was also engineered for ease of genetic manipulation; for example, the insertion of a murine ecotropic viral receptor for efficient retroviral transduction. This, combined with their immortalized and non-transformed state, make HMF3A a useful platform for cell engineering and fibroblast biology, as well as breast tissue microenvironment modeling in stroma-epithelial interactions or age-related mechanisms.
CBFB-Rich Exosomes Promote the Acquisition of a CAF-Like Phenotype
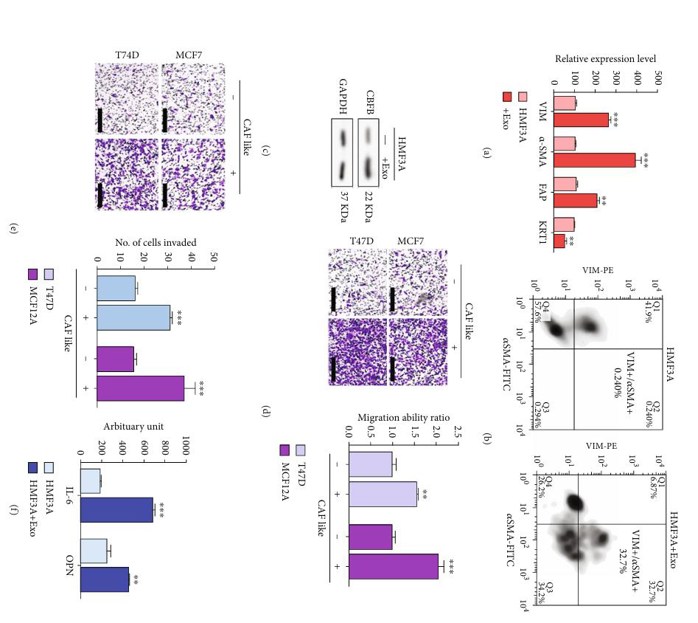
Metastasis is the leading cause of breast cancer death. Breast cancer cells are known to experience high oxidative stress. Circulating exosomes have a variety of roles in tumorigenesis and metastasis. Hsu et al. studied CBFB and its role in breast cancer bone metastasis and how it might affect oxidative stress-related targets. In this study, HMF3A cells cocultured with exosomes demonstrated a significantly higher expression of the CAF markers vimentin, α-SMA, and FAP, and a lower cytokeratin 1 (KRT1) expression (Fig. 1a). Flow cytometry also showed a significant increase in the proportion of CAF-like (VIM+/α-SMA+) cells in HMF3A exosome cocultured cells compared to those not cocultured with exosomes (Fig. 1b). Western blot analysis revealed higher CBFB expression in HMF3A cells cocultured with exosomes (Fig. 1c). These results show that exosomes are rich in CBFB and that CBFB is the driver for the CAF-like phenotype in HMF3A cells. After HMF3A cells were made CAF-like through coculture, they were cocultured with T47D or MCF12A cells. Cocultured HMF3A cells significantly increased the migration and invasion (Fig. 1d and e). Exosome-cocultured HMF3A cells secreted significantly more IL-6 and OPN into the culture medium than singly cultured HMF3A cells (Fig. 1f).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

