
Immortalized Human Dopaminergic Neuronal Precursor Cells (LUHMES)
Cat.No.: CSC-I9136L
Species: Homo sapiens
Source: 8-week-old fetal human ventral mesencephalon
Morphology: Neuronal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT003 HT® Lenti-SV40T Immortalization Kit
LUHMES is an immortalized human dopaminergic neuronal precursor cell line, originally derived from the ventral mesencephalon of an 8‑week‑old embryo. Immortalization was achieved by introducing a tetracycline‑regulated v‑myc transgene and hTERT expression, and this cell line can therefore be induced to robustly proliferate in the presence of doxycycline and to undergo rapid and synchronized differentiation upon removal of doxycycline.
Maintained under defined conditions (advanced DMEM/F12 supplemented with N2, bFGF, and doxycycline), the cells will proliferate as small, adherent round cells; after removal of doxycycline and addition of dbcAMP and GDNF they will rapidly differentiate within five days into mature, highly branched neurons with >99 % conversion efficiency. LUHMES cells express classic dopaminergic markers (TH, AADC, DAT, and VMAT‑2) and have functional dopamine synthesis, release, and reuptake. They generate spontaneous action potentials and have voltage‑gated Na⁺ channels as characteristic for mature neurons. The cells are sensitive to a range of neurotoxins such as MPP⁺ and are thus a useful model system for Parkinson's disease, high‑throughput drug screening, and mechanistic studies of dopaminergic neurodegeneration. Additionally, LUHMES cells are useful for the study of viral latency (e.g. HSV‑1) and are amenable to CRISPR‑mediated gene editing. In summary, LUHMES cells provide a human neuronal platform for addressing a variety of questions in neuronal development and metabolism as well as the function of disease‑associated genes.
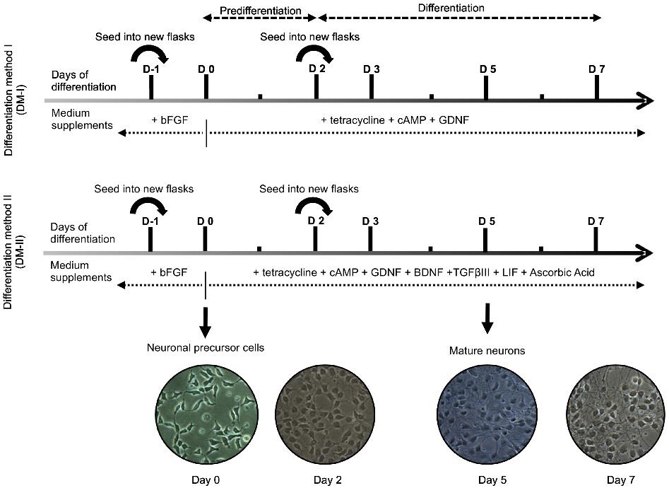
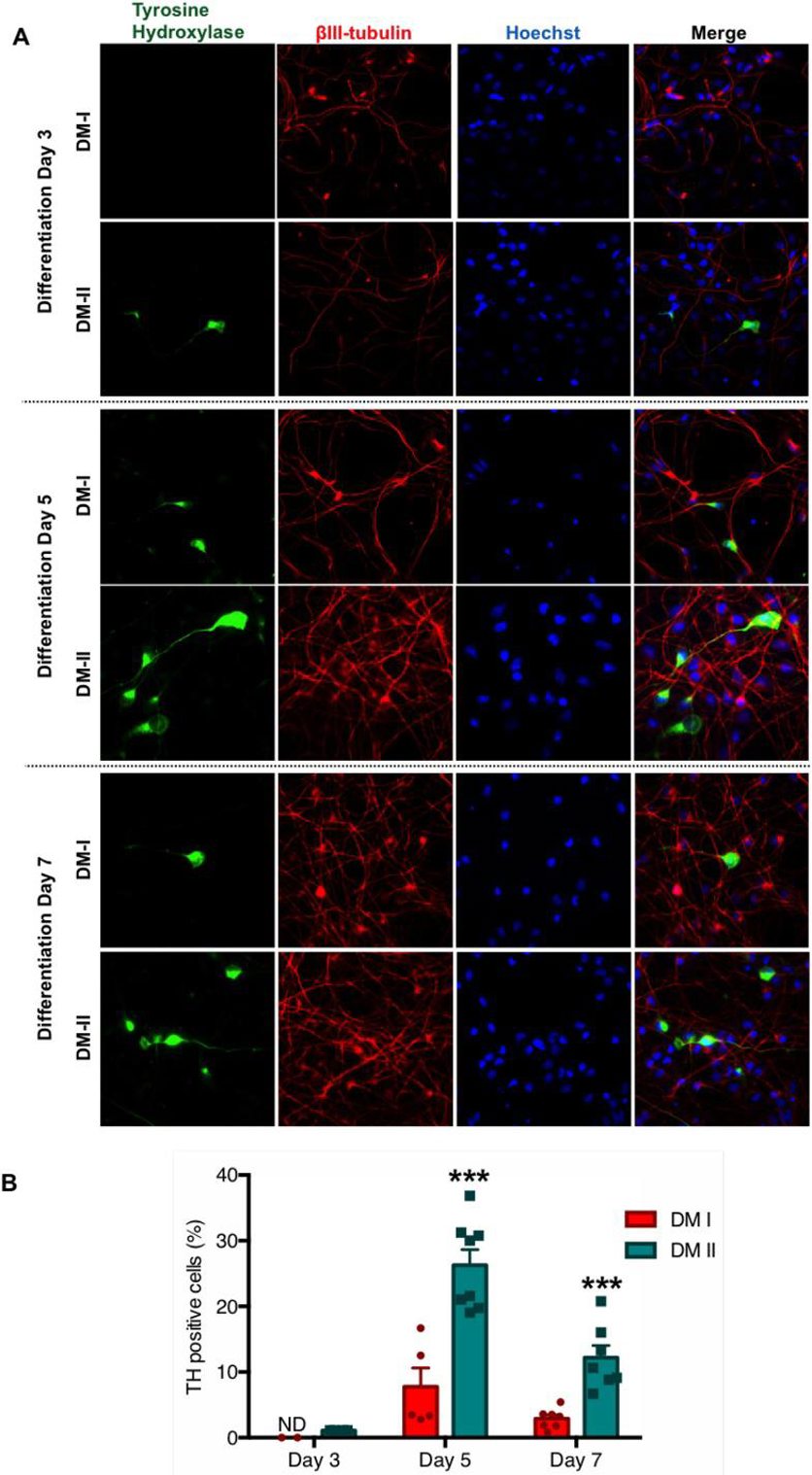
Improved LUHMES Differentiation Protocol Generates More TH-positive Neurons
Human-derived neuronal cell lines, particularly the LUHMES cell line, are valuable for neurobiology and preclinical research. The LUHMES cell line can differentiate into post-mitotic neurons but currently only 10% of cells express tyrosine hydroxylase (TH), a marker for dopaminergic neurons. To address this, Harischandra et al. optimized a differentiation protocol using a cocktail of neurotrophic factors, cytokines, and antioxidants.
The initial LUHMES maintenance and differentiation protocol by Scholz et al. used 40 ng/mL bFGF for proliferation and 1 µg/mL tetracycline for differentiation (Fig. 1, top panel). Our DM-I protocol used growth factors and dibutyryl-cyclic AMP, yielding about 10% TH+ neurons. After literature review and optimization, they developed DM-II, incorporating BDNF, ascorbic acid, TGF-β III, and LIF (Fig. 1, bottom panel). LUHMES cells from the same passage were differentiated using DM-I or DM-II for up to 7 days. At 3, 5, and 7 days post-induction, cells were probed for dopaminergic markers. The new protocol (DM-II) consistently produced more TH+ neurons over 7 days (Fig. 2A). Quantification showed 1% TH+ cells at day 3 and 25% at day 5 with DM-II, compared to 0% and 7% with DM-I (Fig. 2B). By day 7, TH+ cells decreased to 12% with DM-II and 3% with DM-I.
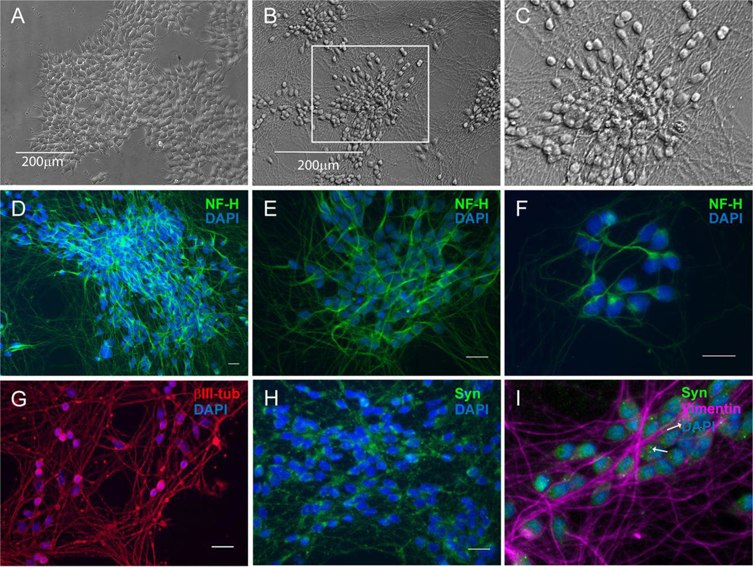
Lund Human Mesencephalic (LUHMES) Neuronal Cell Line Supports Herpes Simplex Virus 1 Latency In Vitro
Lund human mesencephalic (LUHMES) cells are human embryonic neuronal precursor cells that can be differentiated into postmitotic neurons. These cells can be infected with herpes simplex virus 1 (HSV-1), making them a potential model for studying viral latency and reactivation. Unlike rodent neuron cultures, LUHMES cells are scalable and can maintain latency without continuous antiviral inhibitors. Edawrds et al. demonstrated the utility of LUHMES cells as a scalable model for HSV-1 latency and reactivation, mirroring in vivo properties.
The LUHMES cell line, known for its fully differentiated dopaminergic neuron properties, is a valuable tool for studying neurological diseases like Parkinson's disease. Engineered to express v-myc in a tetracycline-regulatable system, LUHMES cells can proliferate and then differentiate upon tetracycline addition. LUHMES cultures display neurite-like projections (Fig. 3A) and can be differentiated to express mature neuron markers within 5 days using tetracycline, GDNF, and dibutyryl cAMP. For our experiments, LUHMES cells were plated on coverslips, differentiated, and analyzed for neuron-specific markers via immunofluorescence. After 7 days, neuronal filament projections up to 200 µm long were observed (Fig. 3B and C). The differentiated cells contained neurofilament H-positive intermediate filaments (Fig. 3D to F), βIII-tubulin (Fig. 3G), and synaptophysin (Fig. 3H and I).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

