
Immortalized Human Corneal Endothelial Cells-SV40T
Cat.No.: CSC-I9205L
Species: Homo sapiens
Source: Descemets′ membrane
Morphology: Cobblestone-like
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
- Documents
Note: Never can cells be kept at -20°C.
Immortalized Human Corneal Endothelial Cells (HCEnCs) represent a transformative in vitro model system designed to overcome the fundamental bottleneck in corneal endothelial research: the absolute scarcity and non-regenerative nature of primary human tissue in vivo.
The corneal endothelium, a monolayer of hexagonal cells on the inner surface of the cornea, is essential for maintaining corneal transparency through its critical "pump-leak" barrier function, actively regulating stromal hydration via ion transport (primarily Na+/K+-ATPase pumps). Unlike most human cells, these cells are terminally differentiated in vivo with minimal proliferative capacity. Primary HCEnCs obtained from donor tissue are extremely limited in number, exhibit rapid senescence in culture, and lose their defining functional phenotype (e.g., ion pump function, tight junction integrity), making long-term or large-scale studies impractical.
Immortalization strategies, typically involving the introduction of SV40 Large T antigen (SV40LT) and/or the catalytic subunit of human telomerase (hTERT), are employed to confer stable proliferative potential while striving to preserve essential functional characteristics. The paramount advantage of well-characterized immortalized HCEnC lines lies in their provision of a sustainable, genetically uniform, and experimentally tractable human model that faithfully replicates key aspects of native endothelium.
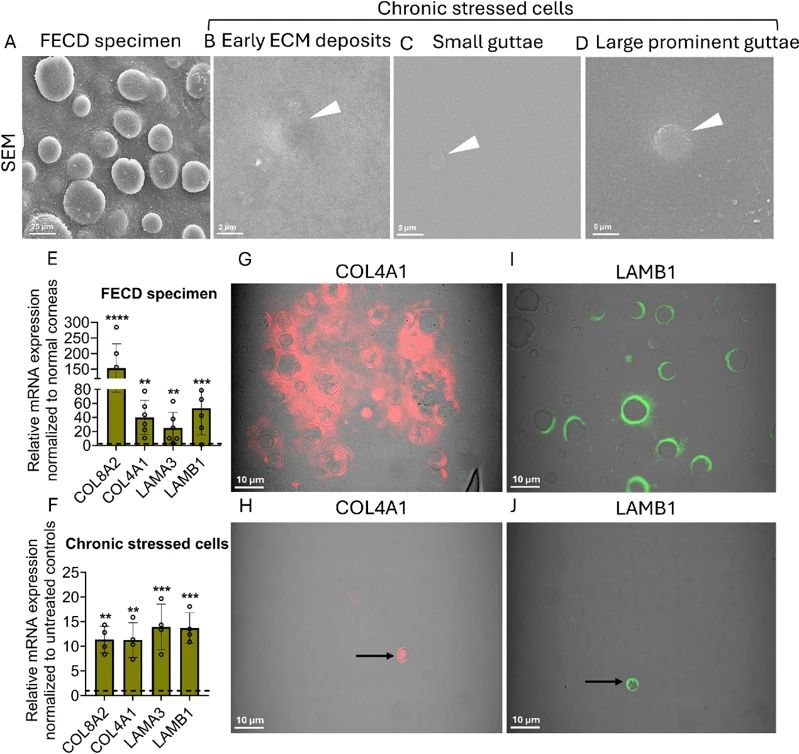
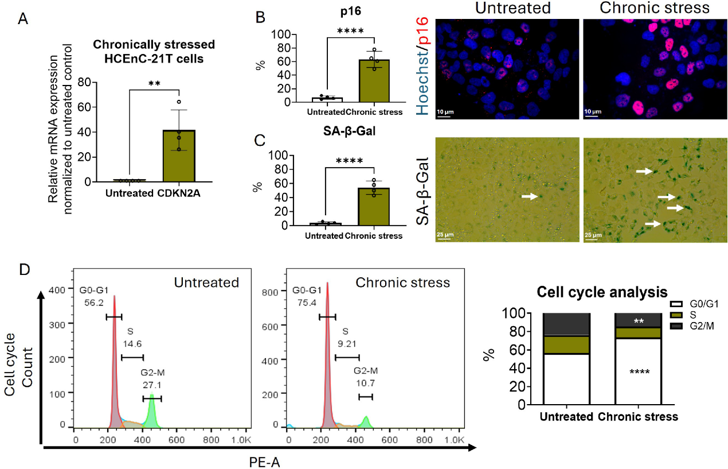
p16-Mediated Senescence Leads to Guttae Formation in An In Vitro Human Corneal Endothelial Cell Model
Fuchs endothelial corneal dystrophy (FECD) is characterized by corneal endothelial cell (CEnC) degeneration and excessive extracellular matrix (ECM) deposition. However, the association between senescence and pathological ECM accumulation remains unclear. This study investigated whether senescence mediated cell-cycle arrest drives aberrant ECM deposition and guttae formation in FECD.
A chronic stress (CS) model was established by concurrently exposing immortalized human CEnCs to ultraviolet-A light (UVA; 25 J/cm2) and 4-hydroxyestradiol (4OHE2; 20 µM) on days 1, 6, and 21, followed by approximately a 60-day recovery period, and the cellular synthesis of guttae-like structures was observed. We also examined senescent-specific cell cycle dynamics and analyzed the conditioned media for secretory factors. Our findings revealed that p16-positive senescent cells that are arrested in G0/G1 phase of the cell cycle secrete ECM components leading to guttae formation. This study presents a biologically relevant in vitro guttae model offering key insights into FECD pathogenesis and a platform for therapies targeting senescence and ECM remodeling.
Immortalized Human Corneal Endothelial Cells-SV40T (Cat No.: CSC-I9205L) are ideal for studying corneal endothelial cell function, wound healing, transplantation research, drug screening, and understanding cellular mechanisms involved in corneal diseases.
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

