
Immortalized Human Chondrocytes-SV40
Cat.No.: CSC-I2082Z
Species: homo sapiens
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
free from contaminations (bacteria incl. mycoplasma, fungi, HIV, HAV, HBV, HCV, Parvo-B19) and cross-contaminations
Note: Never can cells be kept at -20°C.
Immortalized Human Chondrocytes‑SV40 is an in vitro model established from primary human articular cartilage. The cells attach to conventional tissue‑culture plastic and have a polygonal‑to‑fibroblastic morphology. They can be passaged more than 30 times with no decrease in proliferative ability. Immortalized Human Chondrocytes‑SV40 can be maintained in DMEM/F12 supplemented with 10 % fetal bovine serum and express typical markers of cartilage such as collagen II (COL2A1) and aggrecan, as well as a basal level of alkaline phosphatase activity.
In response to pro‑inflammatory cytokines (e.g. IL‑1α, TNF‑α), SV40‑immortalized chondrocytes up‑regulate IL‑6, GM‑CSF and matrix‑degrading enzymes, and can be used to study osteoarthritis pathogenesis and to screen anti‑inflammatory therapeutics. The line is also useful for gene‑editing experiments and tissue‑engineering applications such as scaffold colonization and extracellular matrix deposition, because of its stable genotype and phenotype.
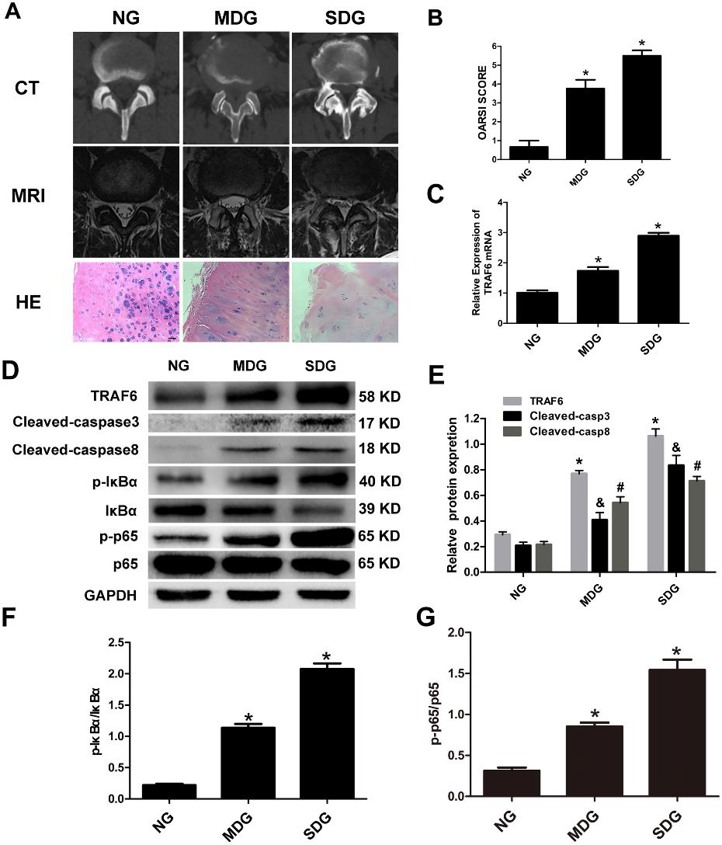
Upregulation of TRAF6 and Apoptotic Markers in LPS-Induced Chondrocytes Apoptosis Model
Tumor necrosis factor receptor-associated factor 6 (TRAF6) is a regulator of NF-κB signaling and may be related to osteoarthritis. However, its function in lumbar facet joint osteoarthritis (FJOA) is unknown. Jiang's team aims to investigate the specific role of TRAF6 in chondrocytes and its connection to FJOA pathophysiology.
In vitro, they stimulated immortalized human chondrocytes by LPS to establish the cells apoptosis model. Based on prior research, they hypothesized that TRAF6 might be involved in FJOA. LPS typically induces inflammation and cell apoptosis. Since chondrocyte apoptosis is a key mechanism in FJOA, they created an LPS-induced chondrocyte apoptosis model and analyzed the link between TRAF6 and apoptosis using caspase-3/8 markers. Figure 1a, b shows that TRAF6 levels rose in chondrocytes over time with LPS stimulation, mirroring the pattern of cleaved caspase-3/8. When treated with the apoptosis inhibitor z-VAD, cleaved caspase-3/8 expression was nearly eliminated, but TRAF6 levels still increased over time (Fig. 1c, d). This suggests TRAF6 is involved in regulating apoptosis via cleaved caspase-3/8. Additionally, ELISA tests showed that LPS increased MMP-13 and IL-6 secretion in chondrocyte supernatants (Fig. 1e-f). Immunofluorescence revealed co-localization of TRAF6 and cleaved caspase-3 in the cytoplasm (Fig. 1g). These findings indicate TRAF6 may significantly impact inflammation and chondrocyte apoptosis in FJOA.
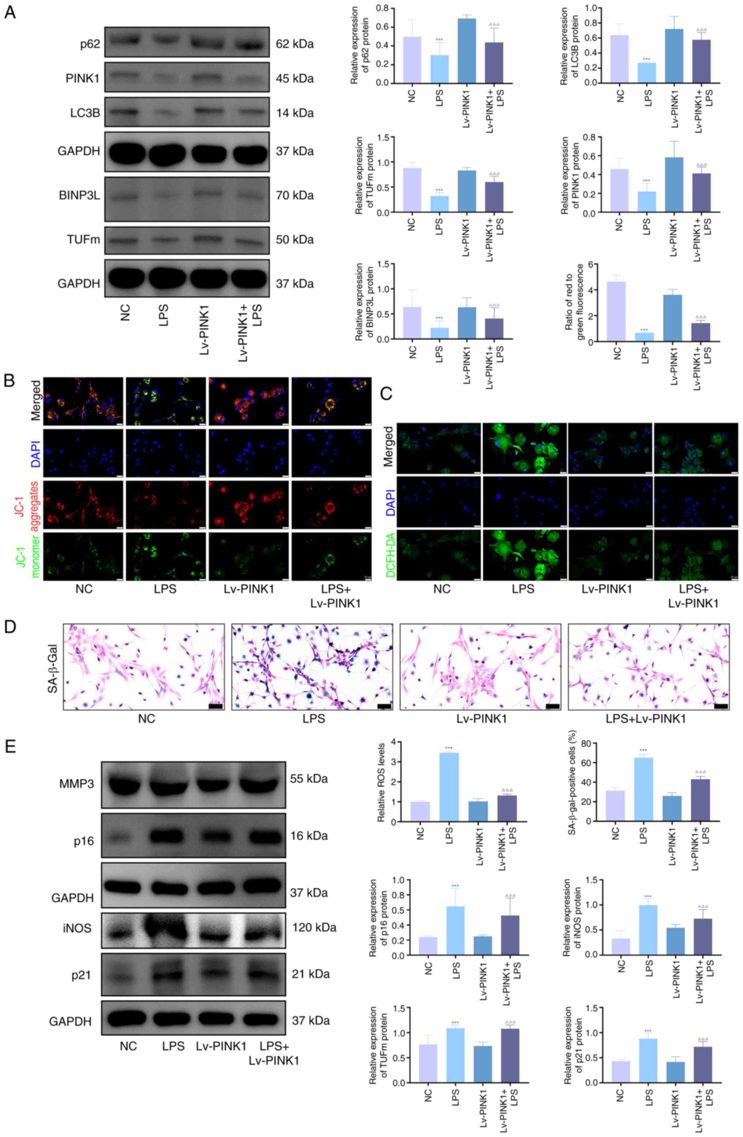
PINK1 Overexpression Decreases Senescence and Promotes Mitophagy in Chondrocytes
PTEN-induced putative kinase 1 (PINK1) is a key regulator of mitophagy and mitochondrial homeostasis, but its role in knee osteoarthritis (OA) is unclear. Jie's team investigated how PINK1 modulates chondrocyte senescence during OA progression.
In vitro, LPS, IL-1β, and TNF-α significantly increased the expression of senescence markers (p21, p16, iNOS, MMP3) and the proportion of SA-β-gal-positive cells. To study PINK1's role in LPS-stimulated chondrocytes, overexpression was achieved using adenoviral transfection. Western blot analysis showed that mitophagy-related proteins (PINK1, TUFm, BINPL, p62, LC3B) were upregulated in KOA chondrocytes compared to controls, but this was reversed by Lv-PINK1 infection (Fig. 2A). JC-1 and ROS fluorescence indicated impaired mitochondrial membrane potential and higher ROS levels in KOA chondrocytes, which were improved by Lv-PINK1 treatment (Fig. 2B, C). SA-β-gal staining revealed increased senescent chondrocytes in KOA, reduced by Lv-PINK1 infection (Fig. 2D). Western blot confirmed decreased senescence-associated proteins after PINK1 overexpression (Fig. 2E). These results show that enhancing PINK1-mediated mitophagy reduces chondrocyte senescence by improving mitochondrial quality control under inflammatory stress.
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

