
Immortalized Human Aortic Smooth Muscle Cells-SV40
Cat.No.: CSC-I9162L
Species: Homo sapiens
Source: Aorta
Morphology: Multipolar
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT003 HT® Lenti-SV40T Immortalization Kit
This cell line was originally derived from primary human aortic smooth muscle cells (SMCs) from tunica media layer of the aorta that are then immortalized by stable transfection with SV40 large T antigen. The cells maintain several important morphological and functional properties of native vascular SMCs, including a spindle-shaped morphology and expression of classic contractile markers such as α-smooth muscle actin (α-SMA), calponin, and SM22α. SV40-mediated immortalization extends lifespan and increases proliferation in these cells compared to primary SMCs while keeping physiological response capabilities intact. This allows the use of this model to study the regulation of vascular tone and extracellular matrix remodeling, as well as disease-associated phenotypic switching of SMCs.
This is an ideal model to study migration, proliferation, and inflammatory responses of SMCs in the context of atherosclerosis, hypertension, and aneurysm formation under controlled experimental conditions. Furthermore, this model can be used as a scalable alternative to primary cells for high-throughput drug screening (e.g., vasoactive compounds, anti-proliferative agents) and mechanistic studies involving TGF-β, PDGF or oxidative stress pathways.
SNHG1 Facilitated Transition of Phenotype VSMCs During Autophagy
Long non‑coding RNAs serve a crucial role in autophagy of vascular smooth muscle cells (VSMCs). Deng's team aims to investigate the effect of small nucleolar RNA host gene 1 (SNHG1) on autophagy in VSMCs and the associated underlying mechanisms.
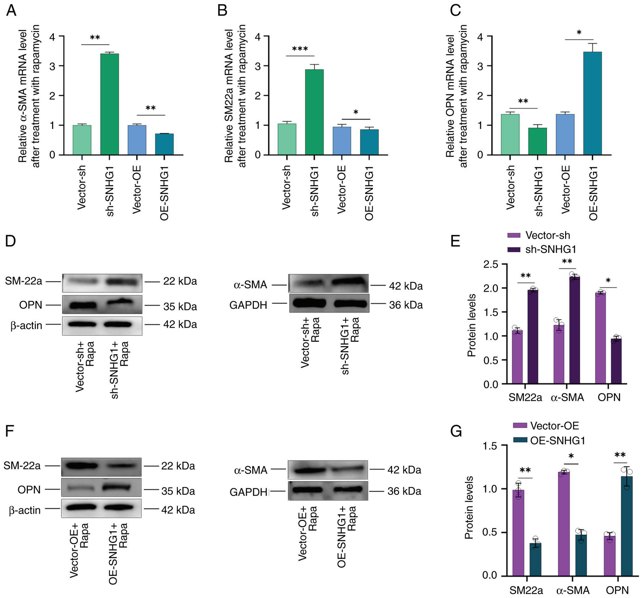
α-SMA, SM22a and OPN are important markers for phenotypic changes following autophagy. When the phenotype of VSMCs shifts from contractile to synthetic, the expression of α-SMA and SM22a decreases, while that of OPN increases. The present study investigated the effects of SNHG1 on phenotypic changes in Immortalized human aortic smooth muscle cells (VSMCs) following autophagy induced by rapamycin at a concentration of 100 nM. It first overexpressed or silenced SNHG1 and then combined treatment with rapamycin in the cells and found that silencing SNHG1 led to an increase in SM22a and α-SMA expression, while OPN expression decreased. Conversely, overexpression of SNHG1 resulted in decreased SM22a and α-SMA expression but increased OPN expression (Fig. 1A-C). Western blotting examination of α-SMA, SM22a and OPN protein levels confirmed these results (Fig. 1D-G). These results indicated that silencing of SNHG1 in VSMCs can induce a shift from a synthetic to a contractile phenotype during autophagy.
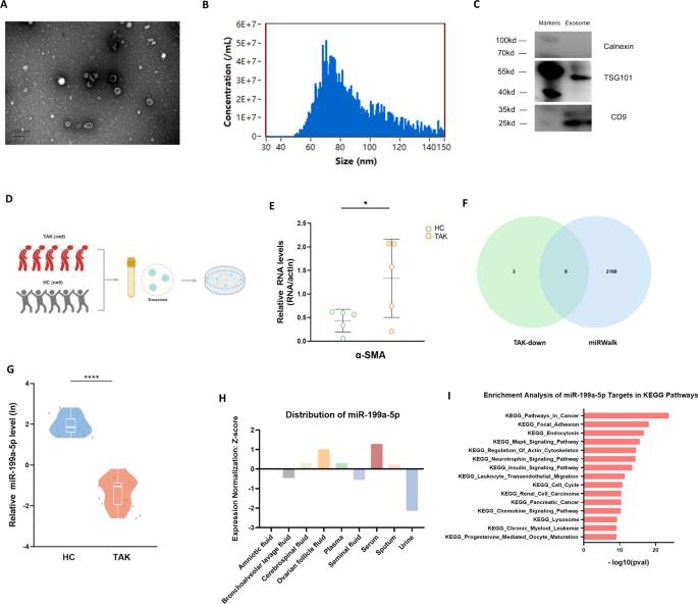
The Serum Exosomes from TAK Patients Contributed to Phenotypic Modulation of VSMC
Takayasu's arteritis (TAK) is characterized by subclinical vascular inflammation and arterial remodeling, with phenotypic switching of vascular smooth muscle cells (VSMC) being a key factor. Exosomes, which facilitate intercellular communication, may play a role in this process. Guo's team explored the regulatory role of serum exosomes in the phenotypic switching of VSMC and vascular remodeling in TAK.
To address whether serum exosomes from TAK patients have an effect on VSMC, they co-cultured human aortic smooth muscle cells with serum exosomes from five TAK patients and five healthy controls (Fig. 2D). qRT-PCR data showed that the VSMC co-cultured with exosomes from TAK patients had higher expression of α-SMA (Fig. 2E), indicating that exosomes participate in phenotypic regulation of VSMC. Then they intersected twelve downregulated miRNAs in TAK exosomes with TAK-related miRNAs from the miRWalk database to obtain nine potential candidate miRNAs (Fig. 2F). Subsequent qRT-PCR results showed that the expression of miR-199a-5p was significantly downregulated in TAK patients (Fig. 2G), while the other eight miRNAs were not significantly different between TAK patients and controls. They then validated the functions of miR-199a-5p using various databases. The levels of miR-199a-5p were high in serum (Fig. 2H), which was indicative that serum exosomes are the main effector of miR-199a-5p. They performed enrichment analysis of mRNAs, and results showed that regulation of the actin cytoskeleton was a major function (Fig. 2I), which was in line with Figure 2E. Taken together, TAK-derived serum exosomes are possibly to regulate the phenotype of VSMC.
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

