Human Kidney Fibroblasts
Cat.No.: CSC-8240W
Species: Human
Source: Kidney
Morphology: Fibroblasts
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can primary cells be kept at -20 °C.
Human kidney fibroblasts (HKFs) are a population of stromal cells that predominate in the renal interstitium and are located around tubules and blood vessels. Fibroblasts are the main source of extracellular matrix, including collagen, fibronectin, and many other structural proteins. Upon renal injury, HKFs rapidly migrate to the damaged area, orchestrating repair and regeneration. Fibroblasts also produce cytokines and chemokines that can affect immune cell recruitment and behavior. In addition, through bidirectional communication with tubular epithelial cells, they can impact physiological and pathologic processes in the kidney. Fibroblast proliferation and excessive extracellular matrix deposition are a major cause of renal dysfunction in chronic kidney diseases. Fibrosis and changes in kidney structure due to unregulated proliferation of fibroblasts are a primary cause of dysfunction and disease progression. Because of this, HKFs are often used as an experimental model for kidney fibrosis.
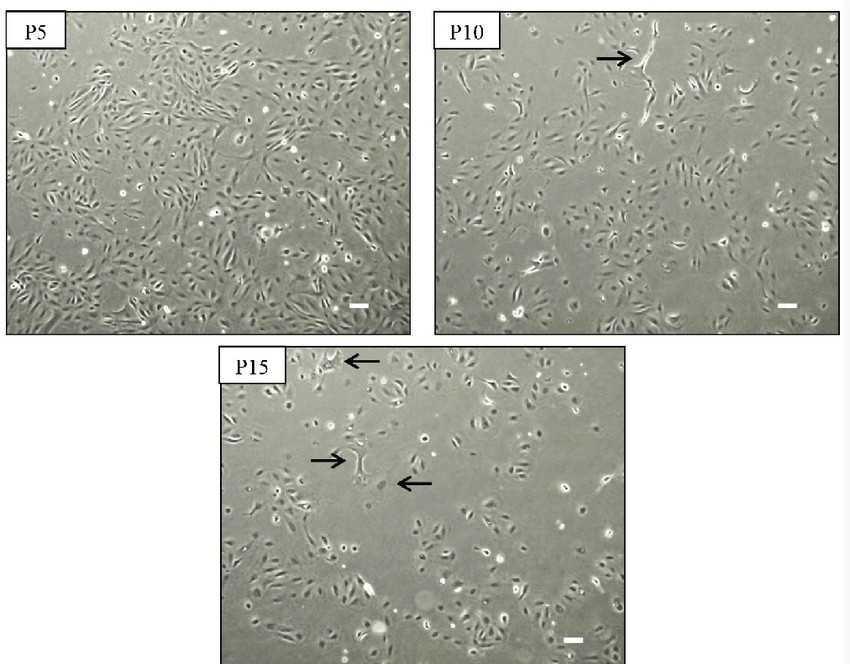 Fig. 1. Morphology of kidney fibroblast cells during serial passages (JENMIT M, Zainuddin Z Z, et al., 2021).
Fig. 1. Morphology of kidney fibroblast cells during serial passages (JENMIT M, Zainuddin Z Z, et al., 2021).
Endoplasmic Reticulum Protein TXNDC5 Promotes Renal Fibrosis by Enforcing TGF-β Signaling in Kidney Fibroblasts
Renal fibrosis remains the final stage of every form of chronic kidney disease (CKD) but lacks any effective treatment options. Chen et al. recently discovered that the ER-resident protein TXNDC5 is a driver of cardiac fibrosis. In the current study, the authors sought to determine whether TXNDC5 is also a driver of kidney fibrosis and whether it can be targeted for treatment.
They then investigated TXNDC5 function in kidney fibroblasts. As expected, TGF-β1 stimulation (10 ng/mL) led to strong induction of TXNDC5 protein (Fig. 1A) and transcript (Fig. 1B) levels in primary human kidney fibroblasts (HKFs), along with the fibroblast activation marker periostin and ECM proteins (COL1A1, fibronectin, and CCN2). TXNDC5 knockdown with shRNA strongly reduced TGF-β1-induced upregulation of the fibroblast activation marker periostin and ECM proteins in HKFs at the protein (Fig. 1A) and mRNA (Fig. 1B) levels. TGF-β1 treatment also increased HKF proliferation, which was completely blocked by TXNDC5 knockdown (Fig. 1D). Thus, TXNDC5 is essential for TGF-β1-induced HKF activation, proliferation, and ECM production. Conversely, TXNDC5 overexpression strongly upregulated periostin, COL1A1, and fibronectin (Fig. 1C) and markedly increased HKF proliferation (Fig. 1E). Together, these findings establish that TXNDC5, acting downstream of TGF-β1, is essential and sufficient to drive kidney fibroblast activation, proliferation, and ECM production.
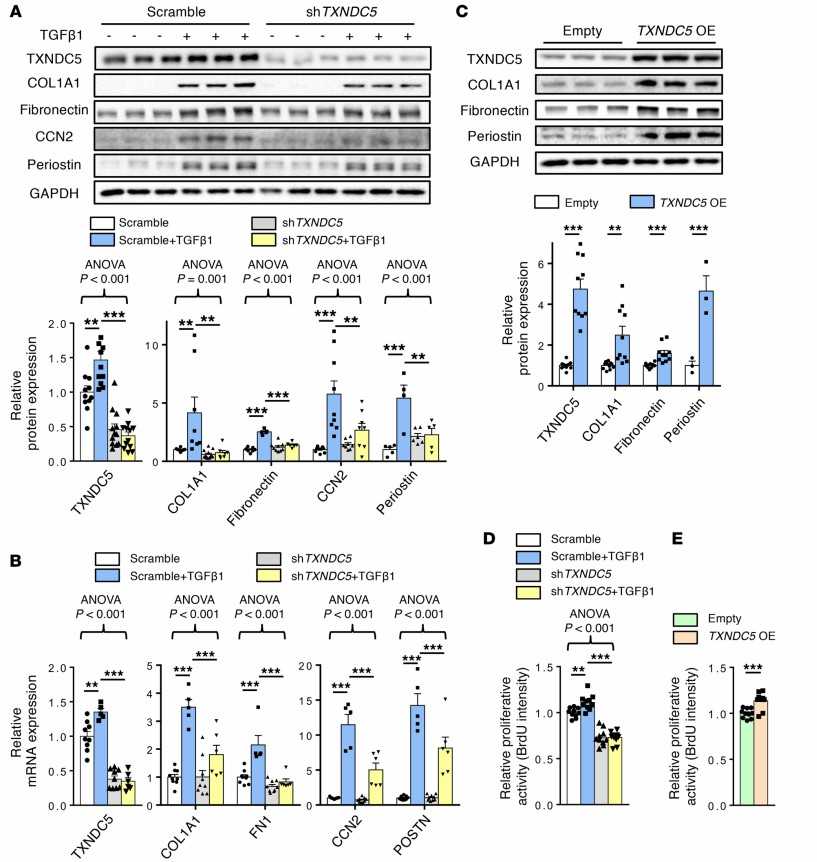 Fig. 1. Knockdown of TXNDC5 attenuated TGF-β1–induced HKF activation and ECM production; overexpression of TXNDC5 was sufficient to trigger HKF activation and ECM production (Chen Y T, Jhao P Y, et al., 2021).
Fig. 1. Knockdown of TXNDC5 attenuated TGF-β1–induced HKF activation and ECM production; overexpression of TXNDC5 was sufficient to trigger HKF activation and ECM production (Chen Y T, Jhao P Y, et al., 2021).
Artesunate Suppressed TGF-β Expression and Alleviated the Activation of the TGF-β/SMAD Pathway in UUO-kidneys and Renal Fibroblasts
CKD inevitably progresses to renal failure with irreversible fibrosis and there is no available antifibrotic therapy. Artesunate has broad-spectrum antifibrotic activities, however, its mechanisms of action in renal disease are not established. TGF-β has been shown to play a critical role in the initiation and progression of kidney fibrosis via canonical and non-canonical pathways. Therefore, Mohammad et al. tested the impact of artesunate treatment on TGF-β expression and the TGF-β/SMAD pathway.
Western blotting revealed a significant upregulation of TGF-β expression and increased SMAD2/SMAD3 phosphorylation in obstructed kidneys. The administration of artesunate suppressed TGF-β expression, and reduced SMAD2 and SMAD3 phosphorylation by 39%, 32% and 44%, respectively (Fig. 2A-D). SMAD3 is a major downstream mediator of TGF-β signaling and a critical driver of fibrosis, which promotes the differentiation of fibroblasts to myofibroblasts and transcription of fibrogenic genes. Western blotting confirmed increased phospho-SMAD3 expression in stimulated fibroblasts, and treatment with artesunate reduced its expression in a dose-dependent manner and restored phospho-SMAD3 expression to control levels at 60 µM (Fig. 2E and F). Gene expression assays demonstrated that artesunate was also able to reduce TGF-β mRNA expression in UUO kidneys. As shown in Figure 2G, artesunate reduced TGF-β mRNA expression by 39% compared to the vehicle-treated group. These results demonstrate that artesunate is able to suppress TGF-β expression, relieve TGF-β/SMAD signaling activation, and repress transcription of pro-fibrotic genes in UUO kidneys and renal fibroblast cultures.
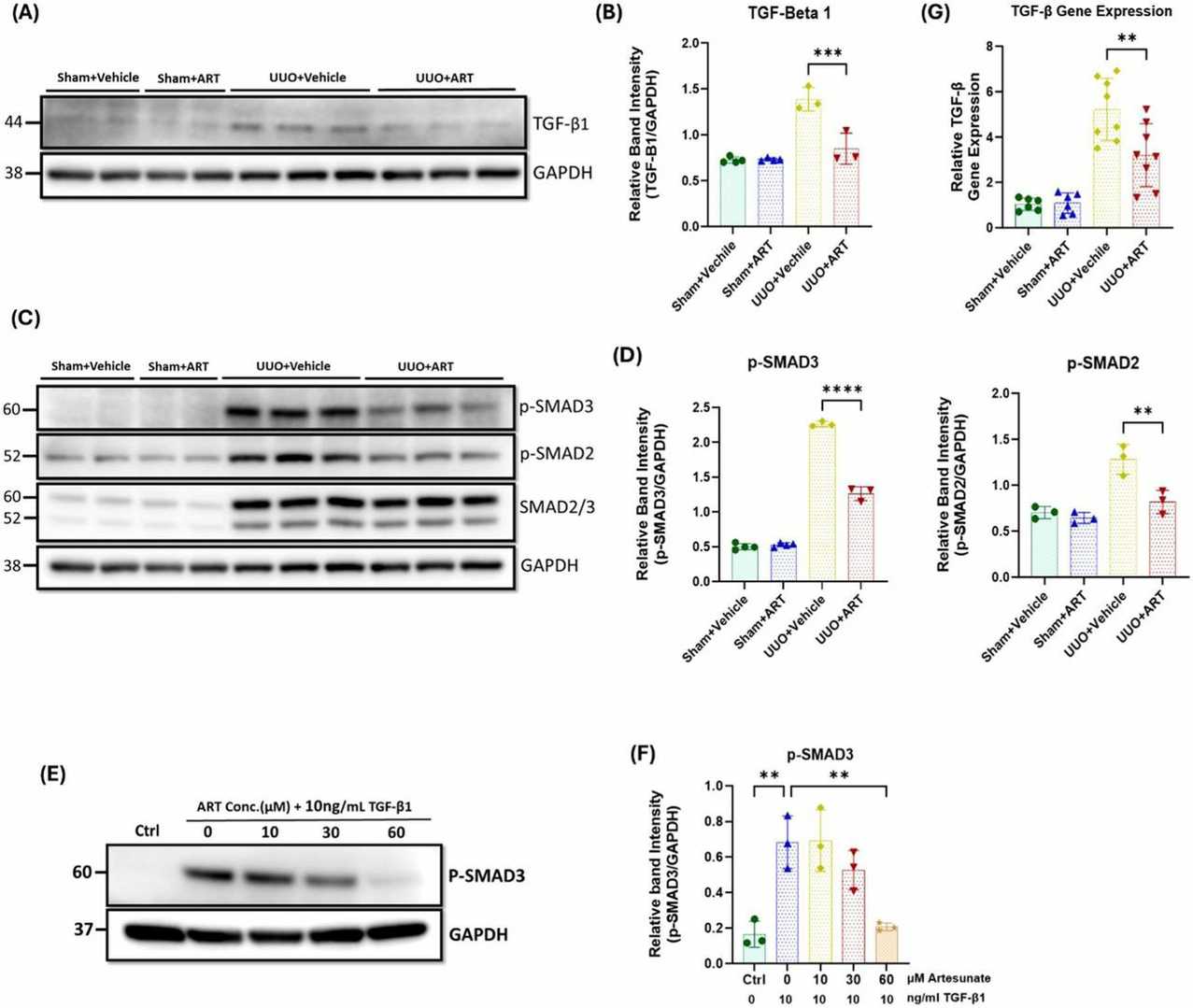 Fig. 2. Artesunate administration reduced TGF-β expression and attenuated TGF-β/SMAD pathway in UUO kidneys and renal fibroblast cell culture (Mohammad G H, Kieswich J, et al., 2025).
Fig. 2. Artesunate administration reduced TGF-β expression and attenuated TGF-β/SMAD pathway in UUO kidneys and renal fibroblast cell culture (Mohammad G H, Kieswich J, et al., 2025).
Ask a Question
Write your own review