Human Kidney Endothelial Cells
Cat.No.: CSC-C8598W
Species: Human
Source: Kidney
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The adult kidney is a highly vascularized organ, notably characterized by a diversity of predominantly quiescent endothelial cell populations lining the blood and lymphatic vessels. The unique microenvironments within the kidney, defined by distinct nephron segments, account for the extensive molecular and functional heterogeneity observed in the renal endothelium.
Specifically, renal endothelial cells (RECs) with distinct phenotype coexist in the three anatomical and functional regions of the kidney, the glomeruli, cortex and medulla — where they support specific kidney tasks. Importantly, technological advances have enabled the study of REC heterogeneity at the single-cell level, providing new insights into their specialized roles in kidney health and disease.
Endothelium in the kidney is unique to that organ and exhibits significant heterogeneity to maintain the kidney's multiple activities in fluid and blood pressure homeostasis. Importantly, REC dysfunction is not only associated with kidney disease but can also contribute to disease progression, suggesting the kidney endothelium as a relevant therapeutic target. Over the past decade, extensive analyses have revealed that metabolism of ECs is tightly regulated at the cellular and subcellular levels, with different EC phenotypes exhibiting unique metabolic profiles. The plasticity of EC metabolism provides a mechanism for orchestrating EC phenotypic behavior, enabling ECs to actively respond to changes in their microenvironment, but also providing a potential opportunity for therapeutic targeting.
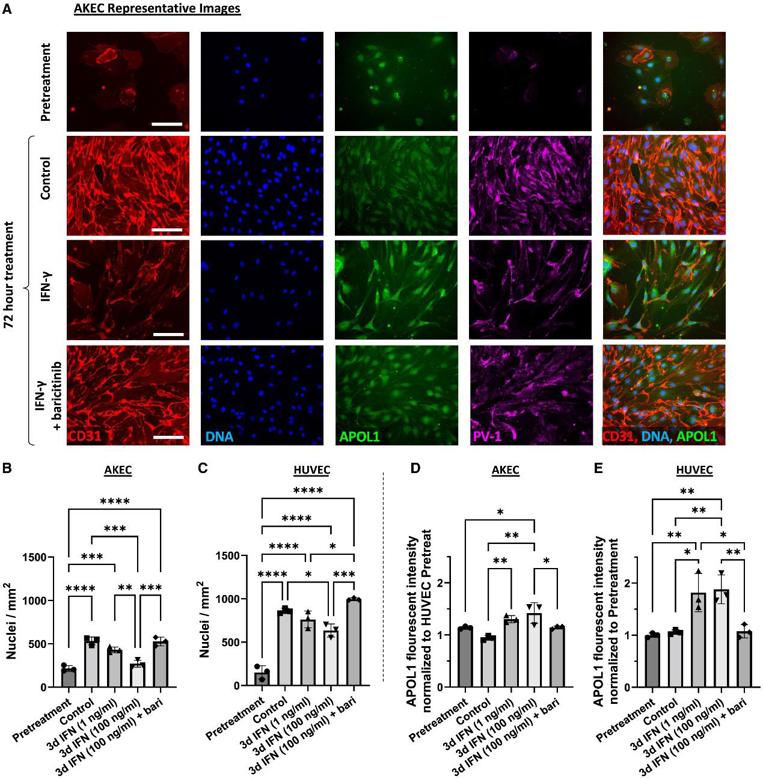
Primary Human AKECs Exhibit Pronounced Sensitivity to IFN-γ
Elevated interferon (IFN) signaling is associated with kidney diseases including COVID-19, HIV, and apolipoprotein-L1 (APOL1) nephropathy, but whether IFNs directly contribute to nephrotoxicity remains unclear. To accomplish this, we compared primary human adult kidney ECs (AKECs) to commercially available human umbilical vein ECs (HUVECs).
When subconfluent cultures were treated with IFN-γ, AKECs exhibited a compact, spindle-like morphology and failed to form confluent monolayers, whereas HUVECs continued to do so (Fig. 1A). Quantitative fluorescence microscopy analysis demonstrated that AKECs treated with 100 ng/mL IFN-γ showed a dramatic dose-dependent decrease in cell number, whereas HUVECs displayed only a modest decrease (Fig. 1B and 1C). To gain further insight into the effect of IFN-γ on AKEC proliferation versus cell loss, AKEC cultures were grown to confluence prior to treatment. With regard to APOL1 expression, HUVECs exhibited a robust response, whereas AKECs exhibited a cell-density dependent effect; subconfluent cultures showed only a modest increase after treatment, but confluent cultures demonstrated robust upregulation of APOL1 (Fig. 1D and 1E). These results suggested endothelial-origin- and cell-density-dependent differences in the anti-angiogenic effect of IFN-γ, and that ECs of renal origin are particularly sensitive to IFN-γ treatment, likely via an APOL1-independent mechanism.
Ask a Question
Write your own review