Porcine Brain Microvascular Endothelial Cells
Cat.No.: CSC-C4204X
Species: Pig
Source: Brain
Cell Type: Endothelial Cell; Microvascular Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Porcine brain microvascular endothelial cells (PBMECs) originate from microvascular endothelial cells extracted from the porcine brain tissue. The structure of PBMECs includes a monolayer formation reinforced by tight junctions and their complexes which serve as essential components to preserve barrier integrity. The main role of these cells involves protecting the blood-brain barrier (BBB) while managing transmembrane transport mechanisms. PBMECs use tight junctions and selectively permeable barriers to prevent large molecules and harmful substances from entering brain tissue while allowing essential nutrients and metabolites to pass through. Additionally, PBMECs produce multiple cytokines and growth factors like platelet-activating factor (PAF) that significantly influence both inflammatory responses and immune regulation mechanisms.
As an in vitro model, PBMECs are widely used to investigate mechanisms of drug transport across the BBB. Researchers use them to investigate both the movement of drug molecules through membranes and how barrier functions are controlled. PBMECs provide a platform for investigating the disease mechanisms behind meningitis and encephalitis. Research shows Streptococcus suis and Haemophilus parasuis pathogens interact with PBMECs to enter brain tissue and initiate inflammatory responses. Furthermore, PBMECs demonstrate therapeutic promise for treating neurodegenerative diseases and neural injuries.
Increased HSPD1 Promotes PBMECs and 293T Cells Apoptosis
Streptococcus suis serovar 2 is highly pathogenic, leading to severe conditions like meningitis in pigs and humans. It penetrates the BBB, often through an interplay with cerebral endothelial cells, via virulence factors such as enolase. Prior research indicates that S. suis drives HSPD1 expression, disrupting BBB integrity and promoting bacterial intrusion, yet the precise pathways remain unclear. Wu's team investigated the tracking of HSPD1 movement from mitochondria to cytoplasm upon Eno interaction, observation of its binding with β-actin, and studying subsequent cellular changes affecting apoptosis regulatory proteins such as Smac, XIAP, and caspase-3.
To evaluate if HSPD1 directly influences Eno-RPSA-induced apoptosis, we overexpressed HSPD1 in 293T cells' cytoplasm. Eno-induced apoptosis was significantly enhanced compared to Eno alone (Fig. 1A). Conversely, reducing HSPD1 with siRNA significantly decreased apoptosis (Fig. 1B), indicating HSPD1's correlation with apoptosis. Also, adding purified HSPD1 to porcine brain microvascular endothelial cells (PBMECs) triggered notable apoptosis, while anti-HSPD1 antibody blocked Eno-induced apoptosis (Fig. 1C and D), suggesting that both intracellular and extracellular HSPD1 promote apoptosis. Previous studies showed that extracellular HSPD1 induces apoptosis via Toll-like receptor 4 (TLR4). Indeed, blocking TLR4 with rabbit anti-TLR4 IgG reduced HSPD1-induced apoptosis (Fig. 1). Overall, these results indicate that HSPD1 is directly linked to Eno-induced apoptosis, with increased HSPD1 expression promoting cell apoptosis.
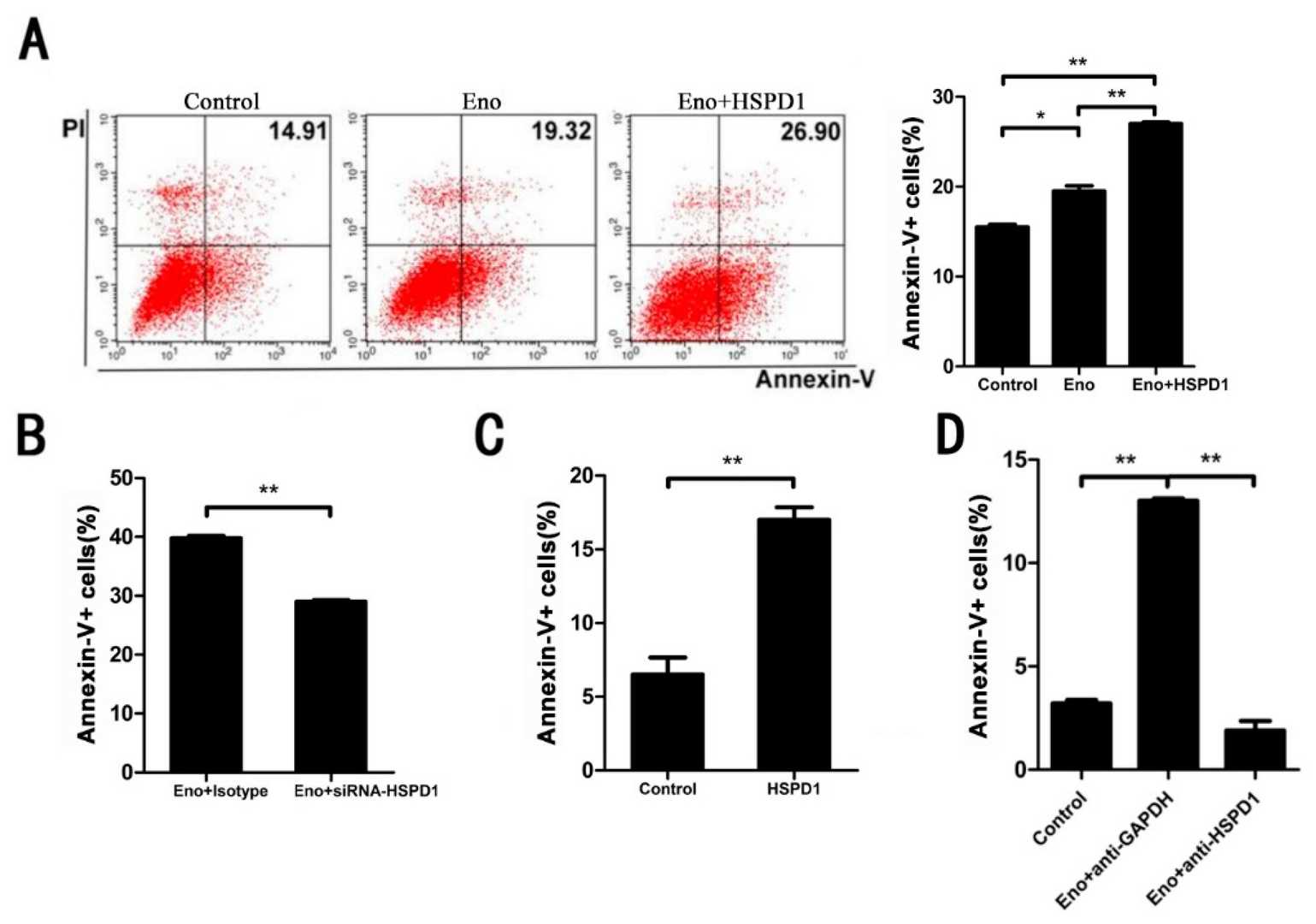 Fig. 1. Both intracellular and extracellular HSPD1 can lead to apoptosis. Flow cytometry analysis was performed to detect Eno- or HSPD1-induced apoptosis (Wu T, Jia L, et al., 2022).
Fig. 1. Both intracellular and extracellular HSPD1 can lead to apoptosis. Flow cytometry analysis was performed to detect Eno- or HSPD1-induced apoptosis (Wu T, Jia L, et al., 2022).
Effect of Amyloid Beta On ATP-Binding Cassette Transporter Expression and Activity in Porcine Brain Microvascular Endothelial Cells
Amyloid-β peptide (Aβ(1–42)) deposition inside the brain serves as a hallmark for Alzheimer's disease. The impact of Aβ(1–42) on BBB ATP-binding Cassette (ABC) efflux transporters which control BBB permeability remains largely unknown. Shubbar et al. assessed how Aβ(1–42) influences ABCB1, ABCC5 and ABCG2 activity and expression as well as transcription factors PXR and CAR in primary porcine brain endothelial cells (PBECs).
Researchers initially employed fluorimetry to measure the activity levels of ABCB1, ABCG2 and ABCC5. The ABCB1 inhibitor verapamil increased intracellular calcein accumulation by seven times (Fig. 2). The ABCG2 inhibitor Ko143 caused a 2.1-fold increase in intracellular Hoechst 33342 concentration while the ABCC5 inhibitor MK571 elevated GS–MF levels by 2.4 times (Fig. 2). The Neutral Red assay confirmed that porcine brain endothelial cells maintaine viability after Aβ(1–42) exposure at concentrations of 10 μg/ml, 25 μg/ml, and 50 μg/ml within a 48-hour period (Fig. 3). The primary research evaluated how Aβ(1–42) formed aggregates within the applied cell culture environment. Thioflavin T fluorescence intensity displayed a significant time-dependent increase when exposed to concentrations of 25 μg/ml and 50 μg/ml of Aβ(1–42) which demonstrated aggregation. The fluorescence intensity at 10 μg/ml concentration remained very low throughout the observation period with no significant changes (Fig. 4).
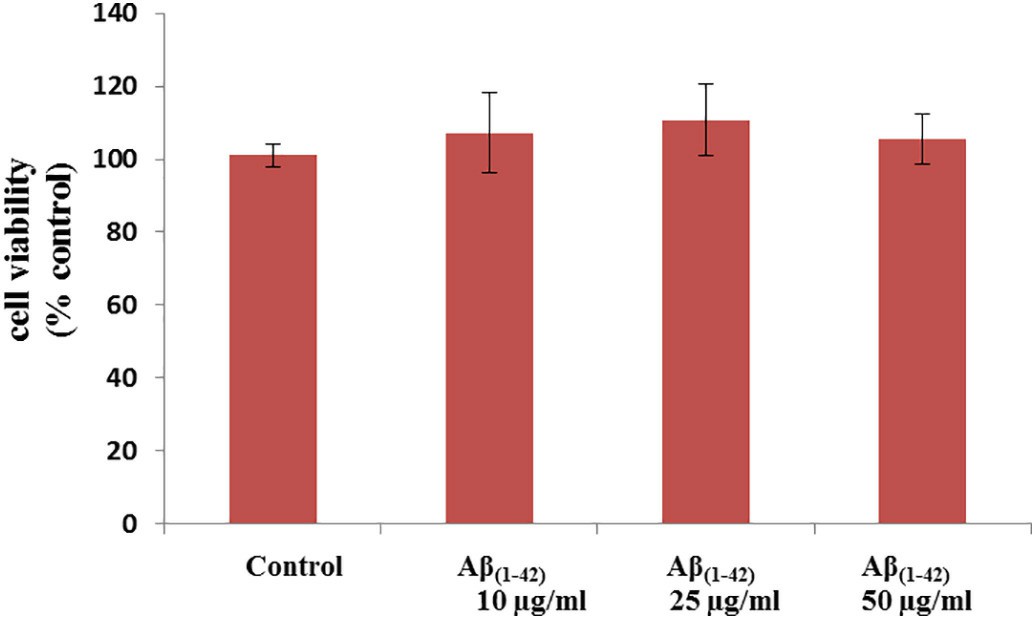 Fig. 2. Determination of ABCB1, ABCG2 and ABCC5 functional activity in PBECs (Shubbar MH and Penny J I, 2018).
Fig. 2. Determination of ABCB1, ABCG2 and ABCC5 functional activity in PBECs (Shubbar MH and Penny J I, 2018).
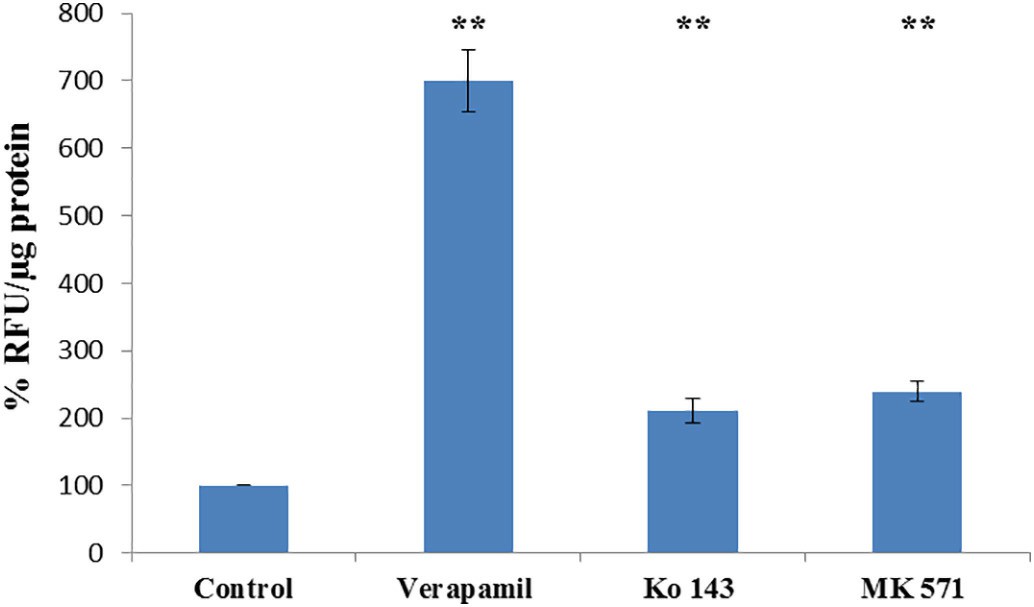 Fig. 3. The effect of Aβ(1–42) on porcine brain endothelial cell viability (Shubbar MH and Penny J I, 2018).
Fig. 3. The effect of Aβ(1–42) on porcine brain endothelial cell viability (Shubbar MH and Penny J I, 2018).
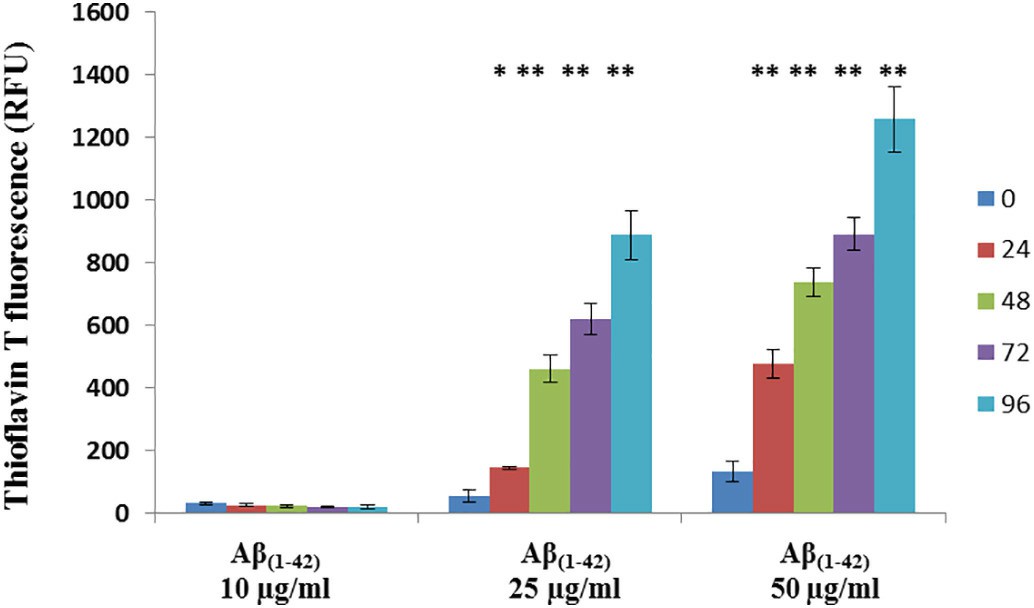 Fig. 4. Aggregation Aβ(1–42) in PBEC treatment medium (Shubbar MH and Penny J I, 2018).
Fig. 4. Aggregation Aβ(1–42) in PBEC treatment medium (Shubbar MH and Penny J I, 2018).
Not possible. Most cell lines contaminated with mycoplasma cannot be distinguished by their appearance except by highly experienced experts.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Great products and services
The development of the company over the years reflects the customers' recognition of the company's products and services.
02 Aug 2023
Ease of use
After sales services
Value for money
Write your own review