L-363
Cat.No.: CSC-C0221
Species: Homo sapiens (Human)
Source: Blood; Peripheral Blood
Morphology: single, large, round or oval cells in suspension
Culture Properties: suspension
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
- Documents
Immunology: CD3 -, CD10 -, CD13 -, CD19 -, CD20 -, CD34 -, CD37 -, cyCD79a -, CD80 -, CD138 +, HLA-DR +,
The L-363 cell line was established in 1977 from the peripheral blood of a 36-year-old woman diagnosed with plasma cell leukemia of the IgG subtype. Plasma cell leukemia is a rare and aggressive form of plasma cell cancer closely related to multiple myeloma.
The L-363 cell line's unique properties, including its EBNA-negative status and its expression of BCL2 proto-oncogene mRNA, have made it a valuable model for investigating the genetic and molecular features of plasma cell leukemia. Researchers have used L-363 cells to explore potential therapeutic targets, drug responses, and mechanisms of disease progression in plasma cell leukemia and related conditions. The insights gained from studies using L-363 cells have contributed to a deeper understanding of plasma cell leukemia and multiple myeloma, leading to advancements in the development of targeted therapies and treatment strategies for these challenging diseases.
Tumor Inhibitory Effects of MicroRNA-124 in Multiple Myeloma Cell Line
Multiple myeloma (MM) is an intricate disease driven by the accumulation of several genetic and epigenetic changes. CDKN2A gene, encoding P16 tumor suppressor and located at 9p21, is dysregulated in several neoplasia by deletions, point mutations, and promoter hypermethylation. The study targeted EZH2 by microRNA-124 (miR-124) in L-363 cells and assessed the following possible impact on CDKN2A gene expression and phenotypic changes.
For stable expression of miR-124, L-363 cells were transduced with lentiviruses by spinoculation protocol which increases transduction efficiency in the presence of 6 mg/ml polybrene (Fig. 1C, D). To evaluate the up-regulation of miR-124 after transduction, the expression level of this microRNA was assessed by qRT-PCR in transduced (as well as non-transduced) L-363 cell lines after 72 hours post-transduction. Comparing the results of pLentiIII- miR-GFP-has-miR-124 and pLenti-III-GFP-mir-control vector-transduced cells with non-transduced L-363 cells, relatively showed respectively about 2.8 ± 0.2 and 87.4 ± 2.4-fold expression changes (Fig. 2).
Flow-cytometric data showed perturbations in pLenti-III-miR-GFP-has-miR-124 transduced cells in comparison with pLenti-III-GFP-mircontrol vector and non-transduced cells. It seems that miR124 overexpression increases the percentage of cells in the G1 phase with a concomitant reduction in the percentage of cells in the S phase (Fig. 3). In line with cell cycle results, MTT assay showed a significant decline in the viability and proliferation of cells with an elevation of miR-124 level (Fig. 4).
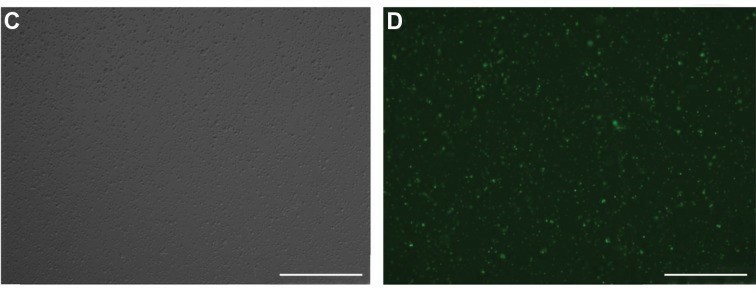 Fig. 1 Light and fluorescent microscopic pictures of L-363 cells 48 hours post-transfection (×10). (Sabour Takanlu J, et al., 2020)
Fig. 1 Light and fluorescent microscopic pictures of L-363 cells 48 hours post-transfection (×10). (Sabour Takanlu J, et al., 2020)
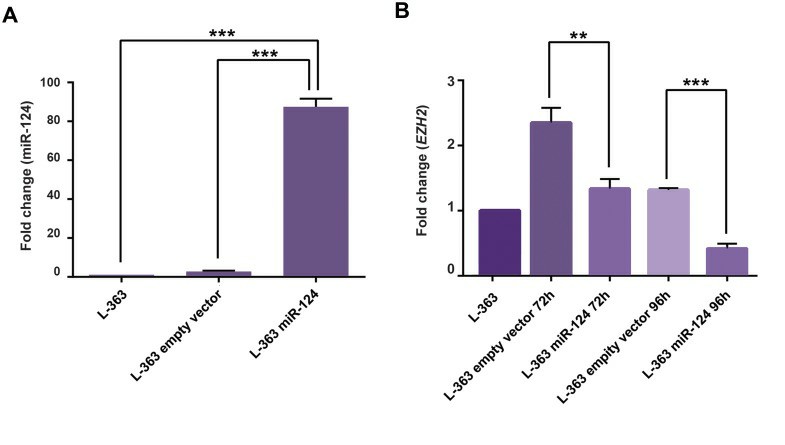 Fig. 2 miR-124 and EZH2 expression fold changes before and after transduction in L-363 cells. (Sabour Takanlu J, et al., 2020)
Fig. 2 miR-124 and EZH2 expression fold changes before and after transduction in L-363 cells. (Sabour Takanlu J, et al., 2020)
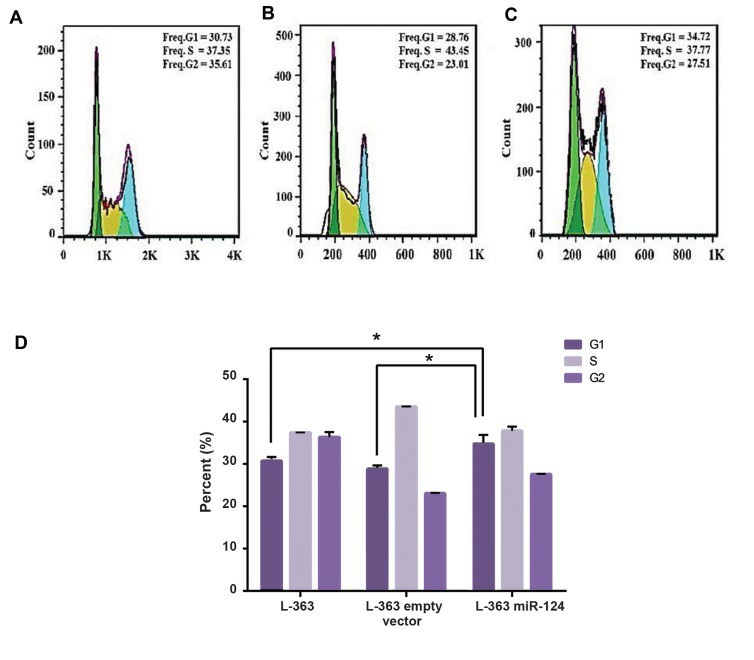 Fig. 3 Cell cycle analysis of L-363 cells before and after transduction of miR-124. (Sabour Takanlu J, et al., 20220)
Fig. 3 Cell cycle analysis of L-363 cells before and after transduction of miR-124. (Sabour Takanlu J, et al., 20220)
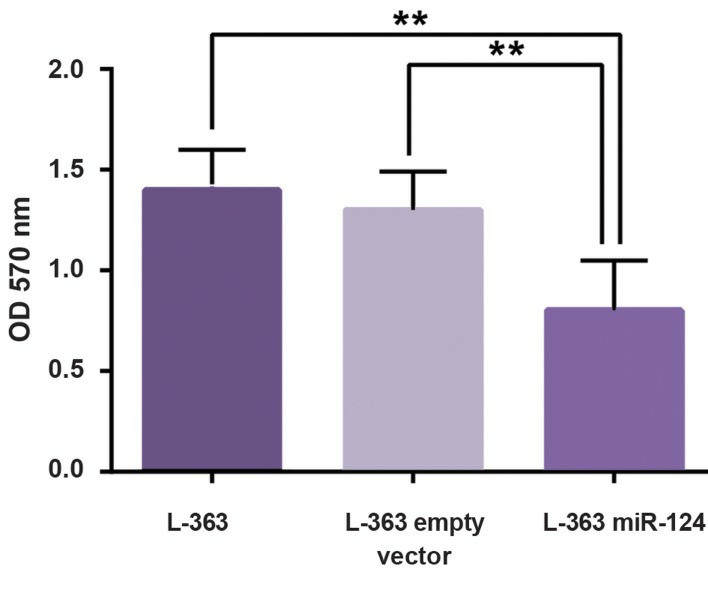 Fig. 4 MTT assay results of L-363 cell after transduction of miR-124. (Sabour Takanlu J, et al., 20220)
Fig. 4 MTT assay results of L-363 cell after transduction of miR-124. (Sabour Takanlu J, et al., 20220)
Roles of Mitochondrial Kv1.3 Channels in Treatment of Multiple Myeloma
Multiple myeloma is a non-curable disease and new therapeutic approaches are needed. PAPTP and PCARBTP, two novel mitochondria-specific inhibitors of the Kv1.3 ion channel, are effective in killing cultured myeloma cell lines and myeloma cells isolated from patient punctuates, while healthy bone marrow cells are not affected.
First, Kv1.3 expression was measured in mitochondria in human myeloma cell lines RPMI-8226 and L-363 and, as the positive control, in Jurkat cells. Pronounced but variable expression of Kv1.3 in both human myeloma cell lines (Fig. 5a). To test the effects of mitochondrial Kv1.3 inhibitors in multiple myeloma cells, the human multiple myeloma cell lines RPMI-8226 and L-363 were incubated with increasing concentrations of PAPTP and PCARBTP (range 0.05 to 10 µM) for 24 h (Fig. 5b). Single treatment with the mitochondria-targeted Kv1.3 inhibitors PCARBTP or PAPTP, respectively, efficiently killed human multiple myeloma cells lines with EC50 ranging between 0.1 and 0.9 µM depending on cell line and inhibitor (Fig. 5b). In detail, PAPTP and PCARBTP efficiently killed the multiple myeloma cell lines RPMI-8226 (EC50 0.1 µM PAPTP/0.26 µM for PCARBTP) and L-363 (0.29 µM/0.87 µM), respectively.
To define the signaling events triggered by PAPTP and PCARBTP leading to cell death, drug-triggered changes were measured in mitochondrial membrane potential, release of reactive oxygen species (ROS), and activation of caspases 3/7. An early increase in mitochondrial ROS and a breakdown of the membrane potential in mitochondria in RPMI-8226 and L-363 cells (Fig. 6a, b). Further, the activation of caspase 3/7 was detected as a hallmark of apoptotic cell death (Fig. 6c).
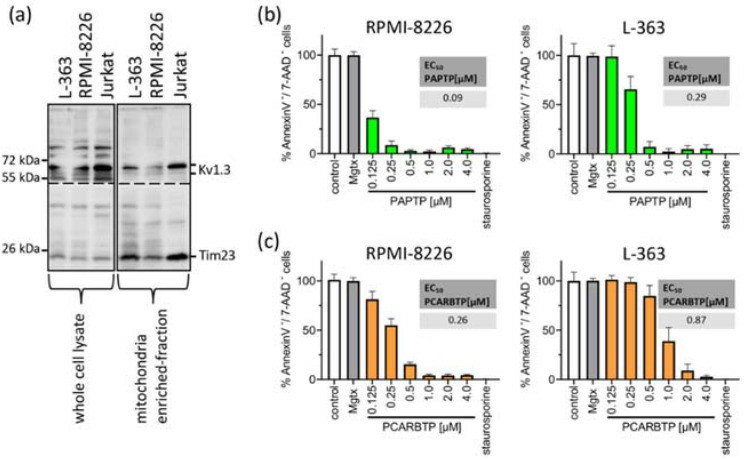 Fig. 5 Mitochondrial Kv1.3 is expressed in the multiple myeloma cell lines L-363 and RPMI-8226. (Kadow S, et al., 2022)
Fig. 5 Mitochondrial Kv1.3 is expressed in the multiple myeloma cell lines L-363 and RPMI-8226. (Kadow S, et al., 2022)
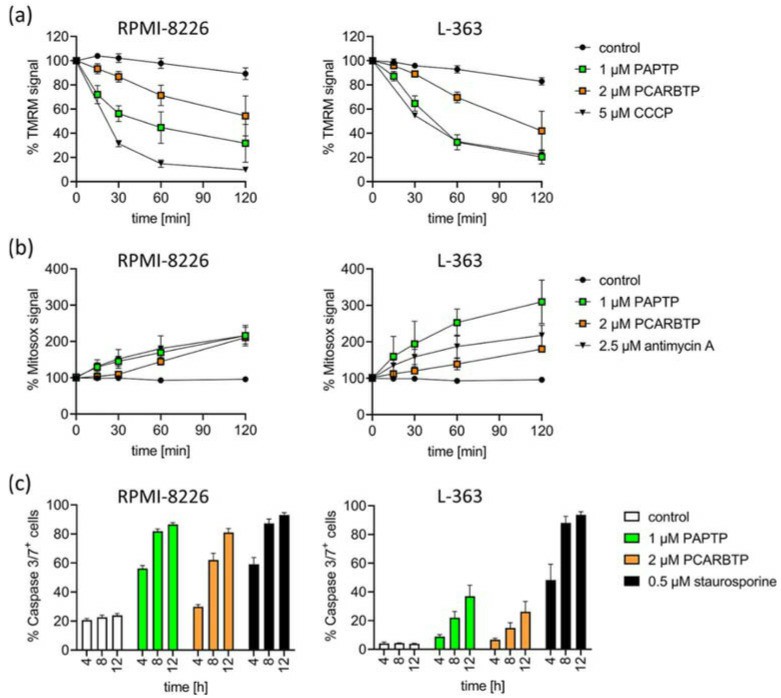 Fig. 6 Inhibition of mitoKv1.3 with PAPTP and PCARBTP triggers apoptosis in human multiple myeloma cell lines L-363 and RPMI-8226. (Kadow S, et al., 2022)
Fig. 6 Inhibition of mitoKv1.3 with PAPTP and PCARBTP triggers apoptosis in human multiple myeloma cell lines L-363 and RPMI-8226. (Kadow S, et al., 2022)
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells