Human Cord Blood CD34+ Cells
Cat.No.: CSC-C8028L
Species: Human
Source: Cord Blood; Blood
Cell Type: CD34+ Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
As these cells mature and differentiate, the levels of CD34 ex
Human Cord Blood CD34+ Cells are a population of hematopoietic progenitor/stem cells (HPSCs) isolated from umbilical cord blood, a rich source of primitive HPSCs obtained post-delivery. They are easier to collect and less immunogenic compared to adult hematopoietic cells and have been used in both clinical and research applications. In vivo, these cells primarily reside in the hematopoietic niche of cord blood. In vitro, they may attach weakly to stromal cell layers or remain suspended in media, depending on the culture conditions. Morphologically, they are small and round with high nuclear to cytoplasmic ratio and sparse cytoplasm with no distinct cytoplasmic granules, and maintain a primitive cellular phenotype. They require media supplemented with hematopoietic growth factors (e.g. SCF, IL-3, G-CSF) for growth. Human Cord Blood CD34+ Cells have a good expansion capacity in vitro. Functionally, they are multi-lineage, capable of differentiating into all mature blood cell lineages (erythrocytes, leukocytes, platelets) and support long-term hematopoiesis.
Human Cord Blood CD34+ Cells are commonly used in hematopoietic stem cell transplantation to treat a range of hematological disorders such as leukemia and thalassemia. They are also used in research applications such as hematopoietic development, gene therapy and drug screening for hematotoxicity. Due to their physiological relevance and accessibility, they are a popular choice in these applications.
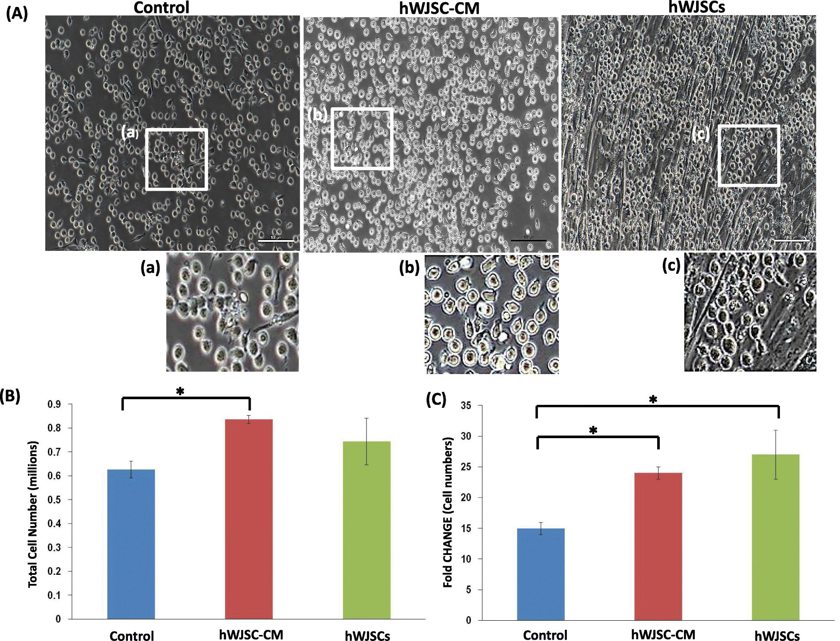
Morphology and Fold Changes of CD34+ Cells Expanded with Hwjscs and HWJSC-CM
Human umbilical cord blood (UCB) CD34+ cells are effective in treating hematological disorders, but their clinical application is limited by low cell numbers. Mesenchymal stem cells (MSCs) in bone marrow act as a scaffold for CD34+ cell proliferation. Lin's team aims to evaluate the use of allogeneic MSCs from human UC Wharton's jelly (hWJSCs) as a stromal support to expand CD34+ cells ex vivo.
The human cord blood CD34+ cells expanded in the presence of hWJSCs had an elongated morphology and pseudopodia-like outgrowths, and migrated towards and loosely attached to the hWJSCs. They showed similar morphologic changes when they were cultured with hWJSC-CM. This behavior was not observed in the controls as the CD34+ cells continued to show their usual circular morphologies (Fig. 1A (a-f)). There were significantly greater viable CD34+ cell numbers after 7 days of culture with hWJSC-CM. The viable cell counts using trypan blue staining were hWJSCs, 0.74 ± 0.10 × 106; hWJSC-CM, 0.84 ± 0.02 × 106; and controls, 0.63 ± 0.04 × 106 (Fig. 1B). The fold change increases were also significant greater with hWJSCs and hWJSC-CM compared to controls. The fold changes normalized to initial starting number of cells were hWJSCs, 27 ± 4; hWJSC-CM, 24 ± 1; and controls, 15 ± 1 (Fig. 1C).
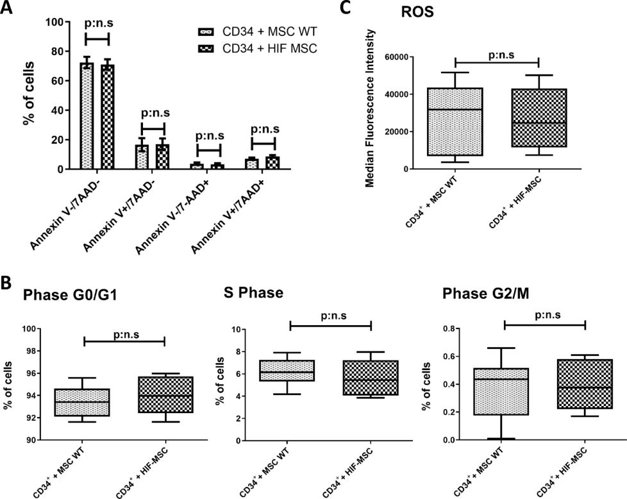
HIF-MSC do not Alter the Viability and Proliferative Capacity of CD34+ Cells
Poor graft function or graft failure after allogeneic stem cell transplantation remains a significant challenge. Mesenchymal stromal cells (MSC) represent a potential treatment strategy due to their angiogenic and immunomodulatory functions. Preciado et al. aim to assess whether co-infusion of human cord blood CD34+ cells with MSC overexpressing hypoxia-inducible factor-1α (HIF-MSCs) may promote hematopoietic stem cell engraftment and function in vitro and in vivo.
They set an in vitro co-culture experiment to evaluate the effect of HIF-MSC in CD34+ cells. Cell viability assays of CD34+ cells were performed after 72 h of co-culture with MSC (n = 10). 78.74% [60.64%-81.66%] of CD34+ cells were viable when co-cultured with MSC WT, with no significant differences with the co-culture with HIF-MSC (75.78% [62.61-77.75%]) (Annexin V-/7-AAD-). There were also no significant differences in the percentage of early apoptotic cells (Annexin V+/7-AAD-), late apoptotic cells (Annexin V+/7-AAD+) and dead cells (Annexin V-/7-AAD+) between both groups (Fig. 2A). Regarding cell cycle, both CD34+ cells co-cultured with MSC WT or HIF-MSC show similar profiles. They did not find significant differences in the percentage of cells in Phase G0/G1 (93.42% [92.11-94.63%] vs 93.97% [92.41-95.72%]), Phase G2/M (0.43% [0.17-0.51%] vs 0.37% [0.22-0.58%]) and S Phase (6.15% [5.30-7.27%] vs 5.44% [40.6-7.22%]) between MSC WT and HIF-MSC, respectively (n = 7) (Fig. 2B).
Ask a Question
Write your own review
- You May Also Need