Kasumi-2
Cat.No.: CSC-C6870J
Species: Homo sapiens (Human)
Source: Bone Marrow
Morphology: Lymphoblast-Like
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Kasumi-2 is a human B-cell precursor acute lymphoblastic leukemia (BCP-ALL) cell line derived in 1990 from the bone marrow of a 15-year-old male patient. Morphologically, Kasumi-2 cells are lymphoblast-like: small, round cells that grow singly or in small clusters, mainly in suspension culture (although some may be loosely adherent). Molecularly, Kasumi-2 cells are characterized by the TCF3-PBX1 (E2A-PBX1) fusion gene, resulting from t(1;19)(q23;p13), a recurrent and clinically significant cytogenetic abnormality in BCP-ALL. Kasumi-2 cells have also been reported to have acquired mutations such as heterozygous HRAS p.Gly13Ser variant. Flow cytometry immunophenotyping has shown Kasumi-2 cells to have the phenotype of a B-cell precursor (CD19⁺, CD10⁺, CD20⁺, CD38⁺, occasional CD34⁺; CD3⁻) consistent with their lymphoid lineage of origin. Given its stable immortalization, well-defined genomics, and known immunophenotype, Kasumi-2 has been used to study the biological effects of the TCF3-PBX1 fusion, investigate gene- and drug-sensitivity, and for omics analyses (genomics, transcriptomics, proteomics), and has been included in resources such as the Cancer Dependency Map and CCLE.
AGO2 Interaction is Essential for Mutant HRAS and NRAS-Driven Cell Proliferation
Activating mutations in RAS GTPases drive nearly 30% of human cancers. Siebenaler et al. investigated the interaction between AGO2 and RAS in both wild-type and mutant HRAS/NRAS cells, exploring its regulation and downstream effects on cell proliferation and senescence.
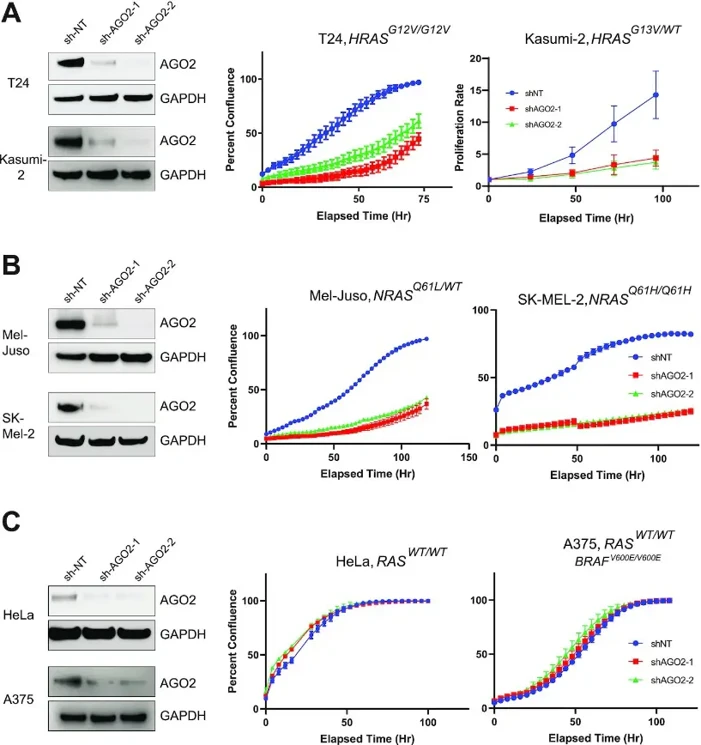
They assessed whether AGO2 functionally promotes mutant HRAS and NRAS cancers. Using two independent shRNAs targeting AGO2, they generated stable cell lines (T24, Kasumi-2, Mel-Juso, SK-MEL-2, H1299, HeLa, and A375) with mutant HRAS or NRAS. As expected, AGO2 knockdown significantly reduced cell proliferation in mutant HRAS-driven T24 and Kasumi-2 cells (Fig. 1A). Similarly, two mutant NRAS-driven melanoma cell lines (Mel-Juso and SK-MEL-2) showed marked growth reduction after AGO2 loss (Fig. 1B). This suggests AGO2 promotes oncogenic HRAS and NRAS proliferation in mutant RAS cells. Their previous work showed that KRAS-independent cell lines are resistant to AGO2 loss. Consistently, AGO2 knockdown in the WT RAS cell line HeLa did not affect cell proliferation (Fig. 1C). Similarly, the BRAFV600E-mutant melanoma cell line A375, which has a constitutively active ERK/MAPK pathway independent of RAS, was also unaffected by AGO2 knockdown (Fig. 1C). Furthermore, overexpression of AGO2 enhanced mutant NrasG12D-driven transformation in NIH-3T3 cells, but did not affect BRAFV600E-driven transformation. These results indicate that AGO2's role in mutant RAS cells is not seen in constitutive MAPK-driven cancers, supporting the necessity of the AGO2-RAS interaction in mutant RAS cells.
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells