Human Pulmonary Vein Smooth Muscle Cells
Cat.No.: CSC-C4859L
Species: Human
Source: Lung; Vein
Cell Type: Smooth Muscle Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
Human Pulmonary Vein Smooth Muscle Cells (hPVSMCs) are human primary cells derived from the tunica media of the pulmonary vein, which is responsible for carrying oxygenated blood from the lungs to the heart. The cell has a spindle shape and tends to have a "hill-and-valley" growth pattern in culture. Human PVSMCs express α-SMA, calponin, and myosin heavy chain as their main markers. They play an important role in regulating vascular tone, extracellular matrix remodeling, and calcium homeostasis. hPVSMCs are often used to study pulmonary hypertension (PH), hypoxia-induced pulmonary vascular dysfunction, and drug responses due to their differing ion channel expression and proliferation mechanisms compared to arterial smooth muscle cells. There are some challenges with donor-to-donor variability and dedifferentiation in long-term cell culture.
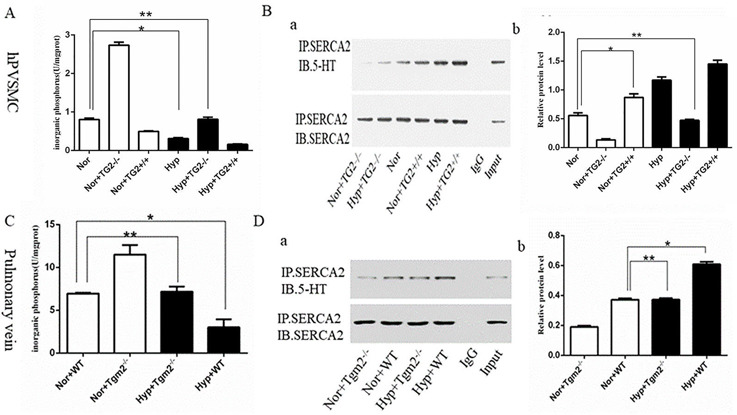
Effect of Hypoxia on the Activity of SERCA2 and Expression of SERCA2 Serotonylation
Sarco-endoplasmic reticulum Ca²⁺ ATPase (SERCA2) pumps are key players in maintaining the balance of intracellular Ca²⁺ in cardiomyocytes and vascular smooth muscle cells (SMCs). Loss or mutation of SERCA2 function causes abnormal calcium signaling, which is associated with the pathophysiology of cardiovascular diseases. Hypoxia is known to disrupt calcium homeostasis, but the role of transglutaminase 2 (TG2) in SERCA2 serotonylation (s-SERCA2) under hypoxia remains unclear.
Liu’s team investigated the effect of TG2 activity on the biological behavior of human pulmonary vein smooth muscle cells (hPVSMC) and by using an inhibitor and agonist of SERCA2, respectively. In vitro experiments showed that the silencing of TG2 promoted organic phosphorus (IP) production, while TG2 overexpression decreased IP production under normoxia. Inorganic phosphorus production was significantly inhibited by hypoxia, and the regulation was dependent on TG2. In contrast to normoxia, the silencing of TG2 did not cause an increase in inorganic phosphorus under hypoxia (Fig. 1A). Co-IP analysis suggested that TG2 overexpression increased s-SERCA2 levels, and the silencing of TG2 decreased s-SERCA2 under normoxia. The expression of s-SERCA2 was significantly enhanced by hypoxia, and this was lost in the silencing of TG2 (Fig. 1B). SERCA2 serotonylation was reported to inhibit the activity of SERCA2. Hypoxia drives the serotonylation of SERCA2 via TG2 in vitro, which underscores the essential role of TG2.
Ask a Question
Write your own review
- You May Also Need