SR-786
Cat.No.: CSC-C0418
Species: Homo sapiens (Human)
Source: Pleural Effusion
Morphology: ovoid-to-round, sometimes irregular, occasionally with apical processes, growing in suspension, singly or in small clumps
Culture Properties: suspension
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Immunology: CD2 -, CD3 -, CD4 +, CD7 -, CD8 -, CD10 -, CD13 -, CD19 -, CD30 +, CD71 +, HLA-DR +, TCRalpha/beta -, TCRgamma/delta -
Viruses: ELISA: reverse transcriptase n
The SR-786 cell line represents a valuable in vitro model for the study of CD30-positive (Ki-1) large T-cell lymphoma, a rare and aggressive subtype of non-Hodgkin lymphoma. This cell line was established in 1983 from the pleural effusion of an 11-year-old boy diagnosed with this specific lymphoma subtype.
The availability of the SR-786 cell line has been instrumental in advancing the understanding of CD30-positive large T-cell lymphoma. Genetic analysis of these cells has revealed the presence of the NPM1-ALK (nucleophosmin-anaplastic lymphoma kinase) fusion gene, a hallmark molecular abnormality observed in a subset of this lymphoma subtype. The NPM1-ALK fusion is known to play a pivotal role in the pathogenesis of this disease, as it leads to the constitutive activation of the ALK signaling pathway, promoting cellular transformation and proliferation.
The SR-786 cell line has been widely utilized by researchers to investigate the molecular mechanisms underlying CD30-positive large T-cell lymphoma, including the role of the NPM1-ALK fusion and its associated signaling cascades. Furthermore, this model system has enabled the evaluation of various therapeutic approaches targeting the ALK kinase and other relevant pathways, with the ultimate goal of improving clinical outcomes for patients with this rare and aggressive form of non-Hodgkin lymphoma.
NOX5 mRNA Expression in ALK+ ALCL Cell Lines
One of the important sources of ROS is NADPH oxidases (NOXs). The isoform NOX5 is highly expressed in lymphoid tissues. In diverse, nonlymphoid malignant cells NOX5 exerts an antiapoptotic effect. Apoptosis suppression is the hallmark feature of a rare type of lymphoma, termed ALK-positive (ALK+) anaplastic large-cell lymphoma (ALCL), and a major factor in the therapy resistance and relapse of ALK+ ALCL tumors.
NOX5 mRNA was strongly expressed in three different ALK+ ALCL cell lines (SR-786, SUP-M2, and Karpas-299). NOX5 is activated by Ca2+ binding through its EF-hand motifs situated in the N-terminus of the molecule. RT-PCR amplification of a 1796-bp part of the NOX5 transcript containing the region of the four EF-hands demonstrated the presence of this crucial activation domain of NOX5 in all ALCL cell lines (Fig. 1A). The N-terminal part of NOX5 can undergo alternative splicing giving rise to different isoforms. The isoforms with the highest expression levels are termed α and β and are present in lymphoid tissues and testis, respectively. In line with their lymphoid origin, all three ALCL cell lines expressed the NOX5α isoform as demonstrated by RT-PCR analysis using isoform-specific primers (Fig. 1B).
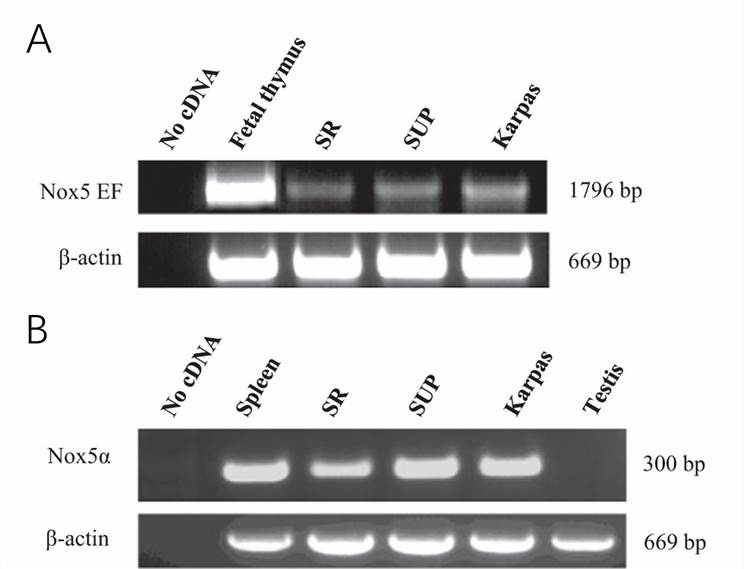 Fig. 1 Unique expression of NOX5 in ALCL cell lines (SR-786, SUP-M2, and Karpas-299). (Carnesecchi S, et al., 2015)
Fig. 1 Unique expression of NOX5 in ALCL cell lines (SR-786, SUP-M2, and Karpas-299). (Carnesecchi S, et al., 2015)
TrkA Binding With NPM-ALK Promotes ALK+ T-Cell Lymphoma Survival
Nucleophosmin-ALK-expressing (NPM-ALK+) T-cell lymphoma is an aggressive neoplasm. ALK may interact with neurotrophic factors. The nerve growth factor (NGF) is the first neurotrophic factor attributed to non-neural functions including cancer cell survival, proliferation, and metastasis. These functions are primarily mediated through the tropomyosin receptor kinase A (TrkA).
Whether inhibition of TrkA may suppress NPM-ALK+ T-cell lymphoma is examined. A small-molecule TrkAi was utilized to selectively antagonize TrkA signaling. Treatment of the lymphoma cells with TrkAi for 48 h caused a concentration-dependent decrease in the levels of pTrkA (Fig. 2A). Data show that at 48 h, inhibition of TrkA was associated with a concentration-dependent decrease in lymphoma cell viability (Fig. 2B). The IC50 was 13.5 μm in SUP-M2, 15.0 in SU-DHL-1, 16.5 in SR-786, and 30.0 in Karpas 299 and DEL cells. Although TrkAi decreased the viability of Karpas 299 and DEL cells, its effects were more pronounced in SR-786, SUP-M2, and SU-DHL-1 cells.
Quantitative evaluation of apoptosis by flow cytometry displayed that inhibition of TrkA caused a significant increase in apoptotic cells (Fig. 2C). In this regard, the effects of TrkAi at 20 μm concentration were more pronounced in the SUP-M2, SR-786, and Karpas 299 cells than in SU-DHL-1 and DEL (Fig. 2C). The effects of the TrkAi were more pronounced in all cell lines at a 30 μm concentration. Giemsa staining showed that TrkAi induced morphologic changes consistent with apoptosis, including cell shrinkage and nuclear condensation and fragmentation (Fig. 2D).
In addition, the BrdU assay demonstrated a TrkAi concentration-dependent decrease in cell proliferation (Fig. 2E). To determine whether TrkA inhibition impedes the colony-forming potential of the NPM-ALK+ T-cell lymphoma, an anchorage-independent colony formation assay was performed. Treatment with the TrkAi suppressed the ability of SR-786, SU-DHL-1, and SUP-M2 cells to form colonies in methylcellulose (Fig. 2F). Representative examples of the colonies from each cell line after seven days from treatment are shown (Fig. 2G).
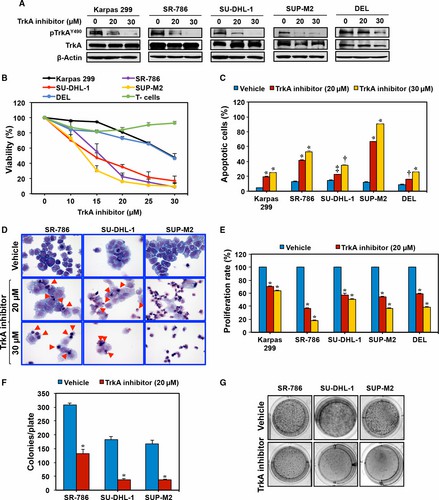 Fig. 2 TrkAi decreases pTrkA levels and induces negative cellular effects in NPM-ALK+ T-cell lymphoma cells. (Shi W, et al., 2017)
Fig. 2 TrkAi decreases pTrkA levels and induces negative cellular effects in NPM-ALK+ T-cell lymphoma cells. (Shi W, et al., 2017)
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells